The photoelectric effect
Electromagnetic radiation is traditionally thought of as a wave. However, Einstein proposed their nature to be particular in his explanation of the photoelectric effect, the emission of electrons from a metal surface when UV light shines on it.
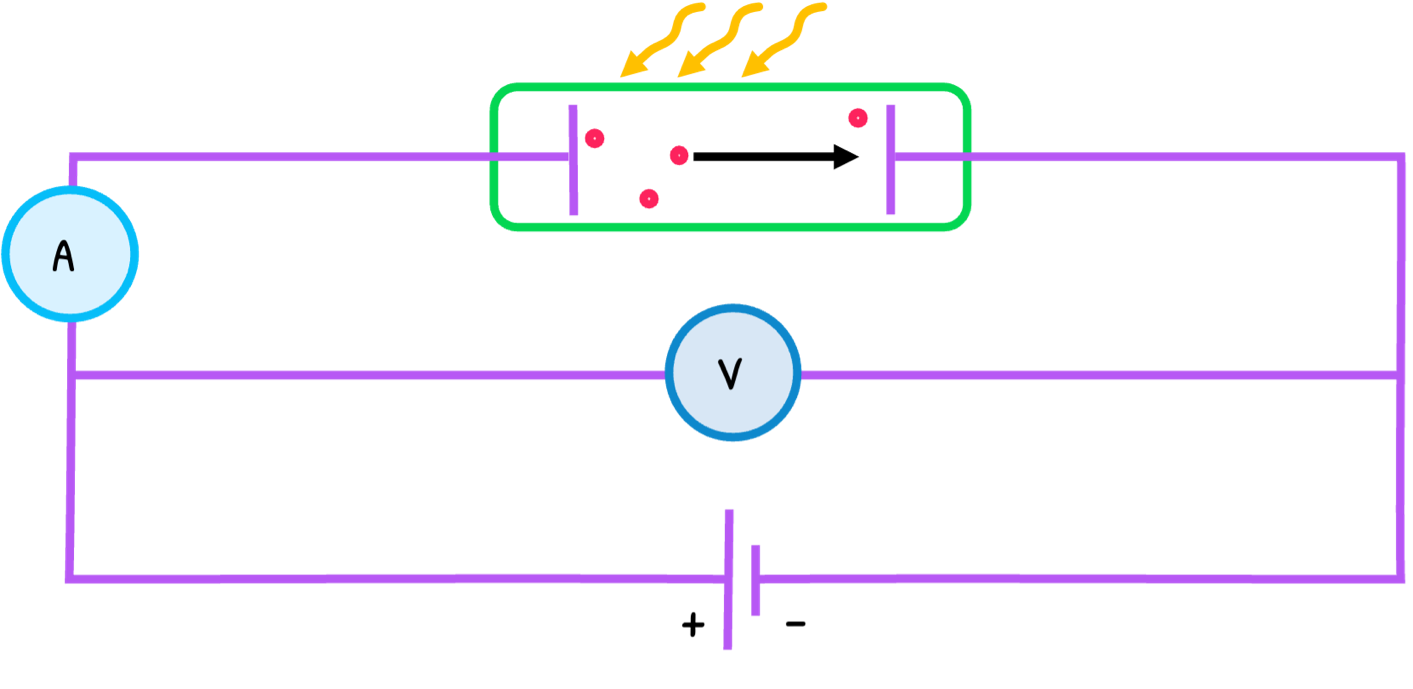
The process is as follows:
- UV light with frequency f arrives in particles called photons. Their energy E is:
E=hf
- The minimum energy that electrons need to escape the energy is called the work function (Φ). Every electron absorbs a photon with a different frequency/energy and if the photon is below a threshold frequency (f0), no electron is emitted.
- If the energy is higher than the work function, the electron escapes and the remaining energy is converted into kinetic energy. The maximum kinetic energy (Emax) is:
Emax=hf−(Φ+ϵ)
- The number of electrons emitted depends on the UV light intensity.
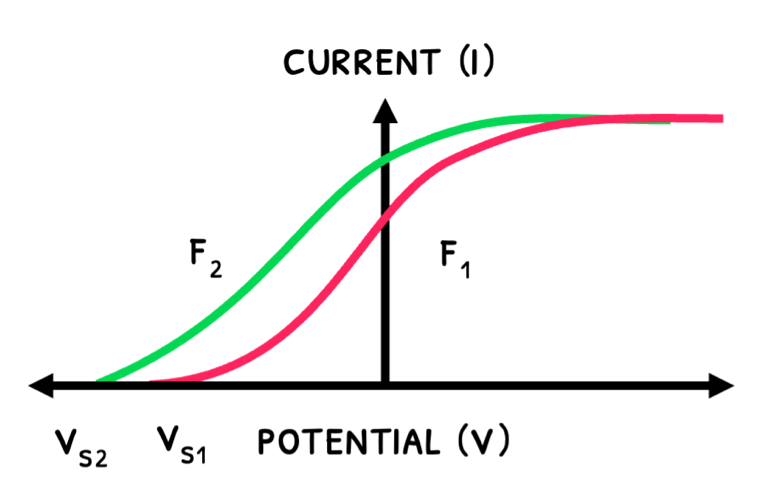
- The process with a reverse supply is known as the stopping potential experiment. In this, the voltage is increased until electrons are decelerated to a point of no emission, called the stopping potential (Vs) and proportional to the electron’s maximum kinetic energy. The equation is:
Ek=Vse
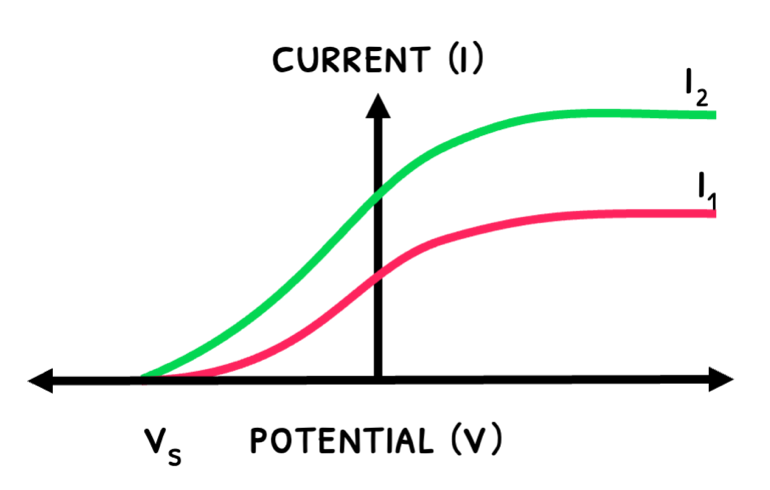
The graphs below show how the current varies with UV light’s intensity and frequency:
- An increase an intensity releases more electrons, increasing maximum current.
- An increase in frequency increases kinetic energy and stopping potential.