Charge
Topic D.2 focuses on the basics of electricity and magnetism. Since electricity is generated by the movement of charged particles, we need to understand what charge is. If you remember, there are two types of charge.
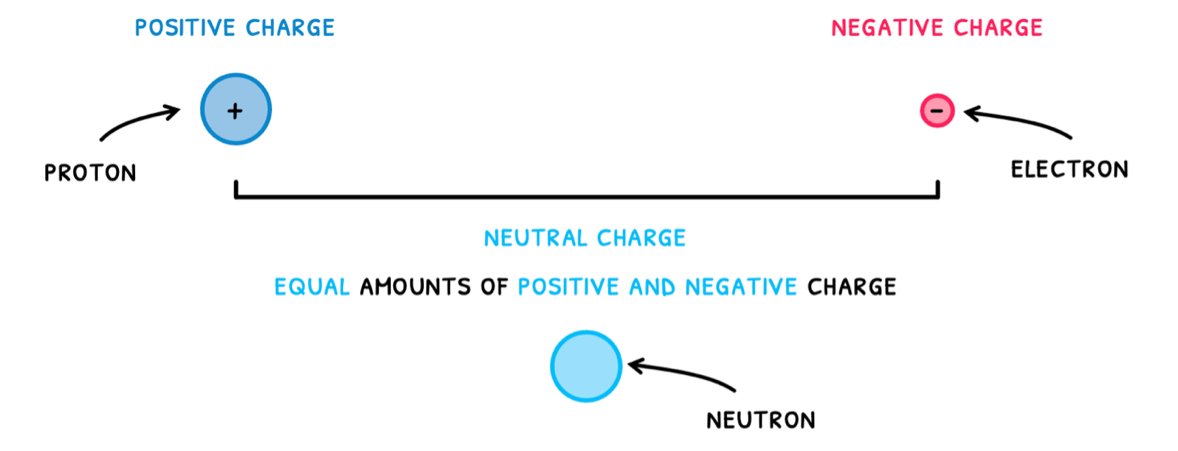
- Positive charge – found in protons
- Negative charge – found in electrons
Neutral charge, found in neutrons, is the presence of equal amounts of positive and negative charge.
Because protons and electrons compose all matter, charge is a fundamental property of all matter. The unit of charge is denoted by q and measured in Coulombs (C), where 1 C = the charge of 6.25 x 1018 particles.
Oil drop experiment
The oil drop experiment was performed by Millikan and Fletcher in 1909 to measure the charge of an electron and begin quantifying charge. This experiment was groundbreaking for quantum physics, eventually leading to a Nobel Prize for Millikan.
The experiment occurred as follows:
- A fine mist of oil droplets is sprayed into a chamber above two metal plates.
- Friction of the oil with the nozzle causes some of the oil droplets to become charged, but this can also be induced via X-ray.
- Oil droplets fall into the space between the two metal plates.
- When the field is turned on, charged droplets move to the plate they are attracted to.
- The oil droplets are observed through a microscope and the voltage is adjusted so only one oil droplet remains.
- When the electric field is turned off, the terminal velocity of the droplet is measured and used to calculate its radius and electric force (F) it experienced.
- The electric field is turned back on, and the charge of the droplet is calculated via the equation:
F=dqV
Performed over many oil droplets, Millikan was able to calculate the charge of an electron (e) to within a standard error of 2% at 1.5924 x 10-19 C. The value you are given in the IB data booklet of 1.60 x 10-19 C was agreed in 2014 and made slightly more precise in 2019, highlighting Millikan’s success at his time.
