Rate Expression
Now that you know basics of rates and the factors that impact it, in the HL syllabus you need to be able to quantify rate and perform calculations.
Let's start by looking at the rate expression, which is used to determine the rate of a reaction at a specific temperature based on the concentration of the reactants. For a two reactant system, the formula is:
Rate=k[A]x[B]y
Note that this is different to a rate equation, which can only be determined with experimental data.
In this:
- [A] is the concentration of reactant A.
- [B] is the concentration of reactant B.
- k is the rate constant.
- x is the order of reactant A.
- y is the order of reactant B.
Whilst [A] and [B] are obvious, the rest are new, so let's cover them.
Rate Constant
The rate constant reflects the rate at a certain temperature, and thus changes as temperature changes. The units used for k depend on the reaction order, so let's cover this.
Reactant Order
For this we start with reactant order, which is the degree to which a reactant's concentration impacts rate. There are four orders to remember:
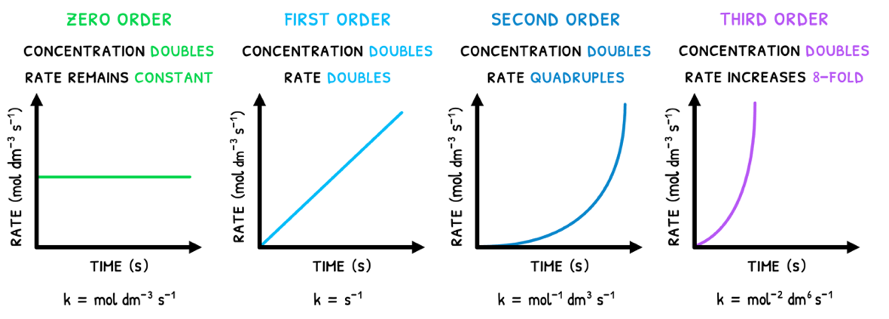
- Zero order - this reactant does not influence rate. Thus, if concentration doubles, rate remains the same.
- First order - this reactant is directly proportional to rate. Thus, if concentration doubles, rate doubles.
- Second order - this reactant is exponentially proportional to rate. Thus, if concentration doubles, rate quadruples.
- Third order - this reactant is cubically proportional to rate. Thus, if concentration doubles, rate octuples.
