Rate of Reactions
Now that you understand atoms, molecular structure, and how energy changes during reactions, you can focus on the reactions themselves. In Topic R2.2, you start this by focusing on the rate of reaction. This is simply the speed at which a reaction occurs and can be thought in a few ways:
- The increase of concentration/mass/volume of products over time.
- The decrease of concentration/mass/volume of reactants over time.
As a result, the units of rate can be mol dm-3 s-1, g s-1 or dm3 s-1. However, mol dm-3 s-1 is the standard unit of measurement for rate.
This is typically represented graphically with volume/mass/concentration on the y-axis and time on the x-axis. In these:
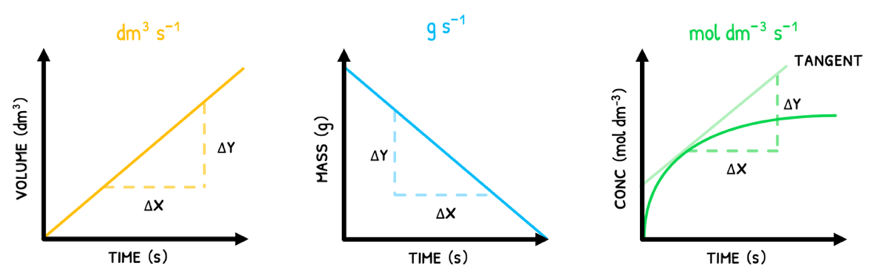
- In straight-line graphs, the rate at any time point is equal to the slope of the line.
- In curved-line graphs, the rate at any time point is equal to the slope of the tangent.
Note that products will show a positive slope because they are produced throughout the reaction whereas reactants will show a negative slope because they are used up throughout the reaction.
Collision Theory
Before learning more about rates, it is essential to understand the basics of molecular movement. Particles in a substance will vibrate or move randomly in space and collide with one another frequently. When certain criteria are met, a collision results in a reaction:
- Correct geometry
- Correct orientation
- Sufficient kinetic energy
Activation Energy
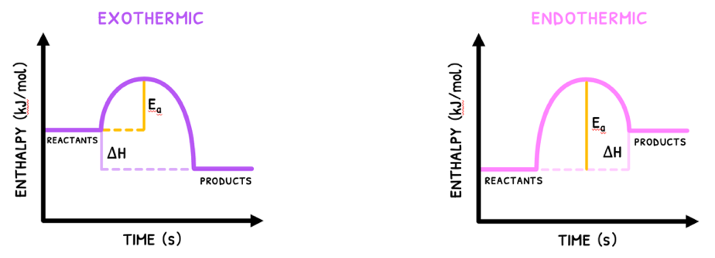
The last criteria is very important, because this is the factor that most determines whether a reaction will or will not occur. It is termed the activation energy (Ea) and is defined as the minimum energy required for two particles to successfully collide and produce a reaction.
Although this may be a new term, it has appeared before in enthalpy diagrams. In these, the curve from reactants to products indicates the activation energy.