IB Chemistry Topic 3 & 13 Notes
S3.1: Periodic table & trends
Periodic table
Dmitri Mendeleev is credited with creation of the first periodic table, which was generally accepted. With minor changes, this became the modern periodic table. In IB chemistry, having a good grasp of the periodic table will help you answer questions quickly and efficiently.
To start, it is important to understand that the periodic table is a table of all known elements. Since every element is distinguished from another by their atomic number (number of protons), they are arranged in order of increasing atomic number.
Then, each row is called a period and each column is called a group. The number of elements in each period and group is dictated by the electron configuration.
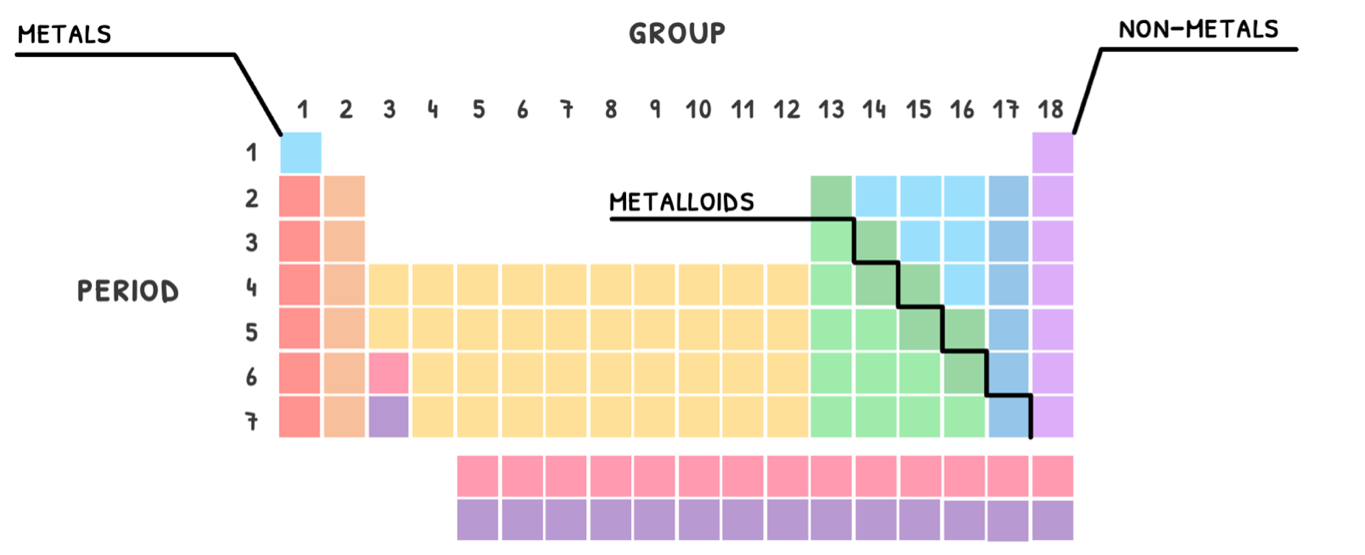
- The period indicates the number of energy levels the element has electrons in.
- The group indicates the number of electrons in the outermost energy level, called valence electrons.
Additionally, this organization also group elements by their properties. As a rule of thumb, the left and middle of the table is classed as metals, the right as non-metals, and in between a line of metalloids.
Periodic table groups
In addition, you are expected to know that since groups share the same number of valence electrons, they typically share the same properties. As such, they are given specific names.
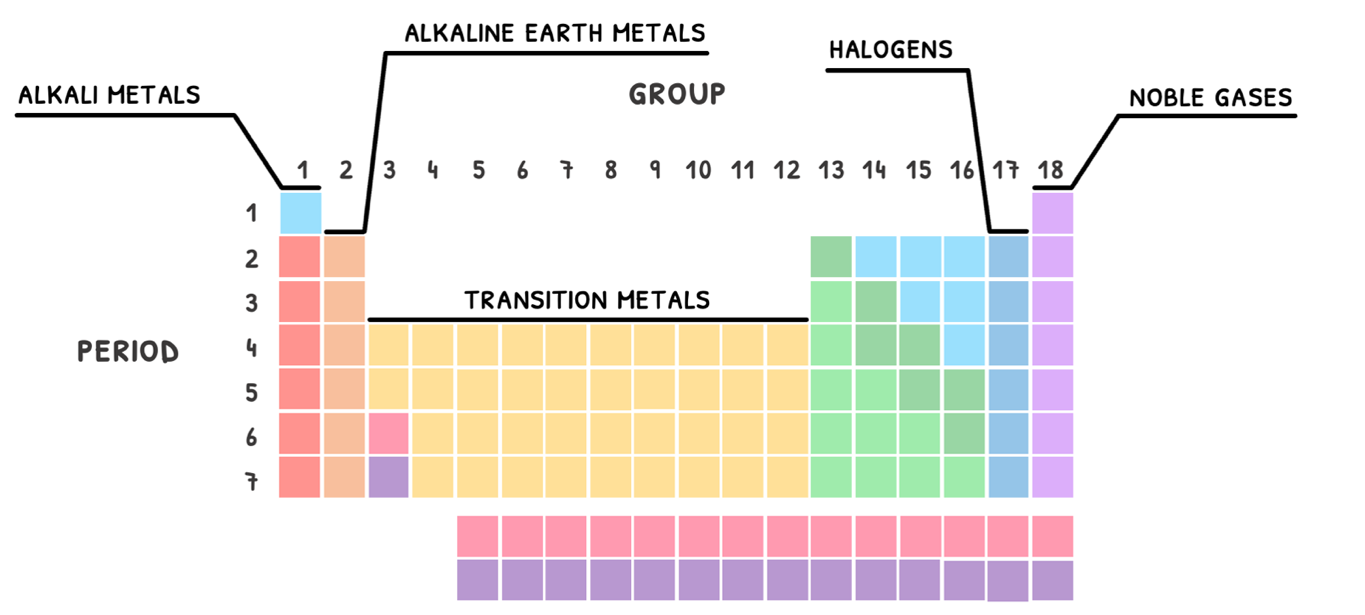
The ones you need to know are summarized in the table below:
| Group | Name |
|---|---|
| 1 | Alkali metals |
| 2 | Alkaline earth metals |
| 3-12 | Transition metals |
| 17 | Halogens |
| 18 | Noble gases |
Each of these groups will share specific properties that will be explored in further topics.
Sail through the IB!
Sail through the IB!
Sail through the IB!
Sail through the IB!
S3.1: Further periodic table (HL)
Ionization energy discontinuities
In the HL syllabus, you are supposed to appreciate that there are discontinuities in some of the periodic trends. The one example you need to understand this in is ionization energy across a period. What you learned is that:
- The number of protons increases, meaning the nuclear charge increases.
- However, there is no added energy level of non-valence electrons, maintaining the shielding effect.
- As a result, the effective nuclear charge increases across a group.
- Therefore, the electrostatic attraction between the nucleus and the valence electrons increases, and thus valence electrons require more energy to pull away.
- This ultimately means there is an increase in ionization energy, as shown below:
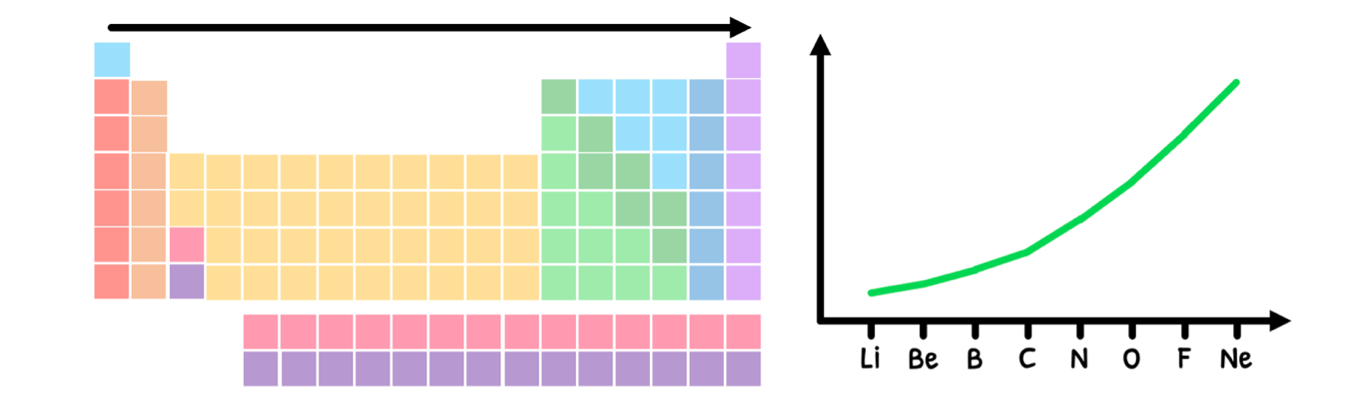
However, the trend is technically not as clean as this graph shows due to the added stability of half-full and full orbitals. Let's look at this in detail:
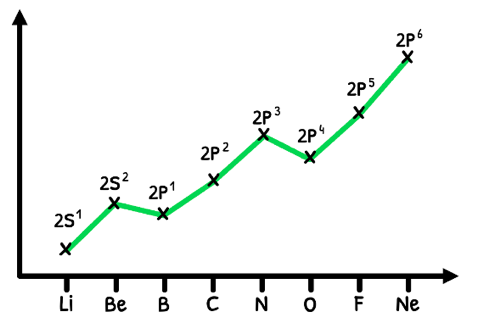
- The first exception, Boron, has the first electron in the 2p orbital. Despite an increase in effective nuclear charge, it is less stable than a full s-orbital so it has a lower ionization energy.
- The second exception, Oxygen, adds the fourth electron to the half-full p-orbital. Despite an increase in effective nuclear charge, the electron-electron repulsion causes instability, decreasing the ionization energy.
Transition metals
HL students also need to know the properties of the first row of transition metals.
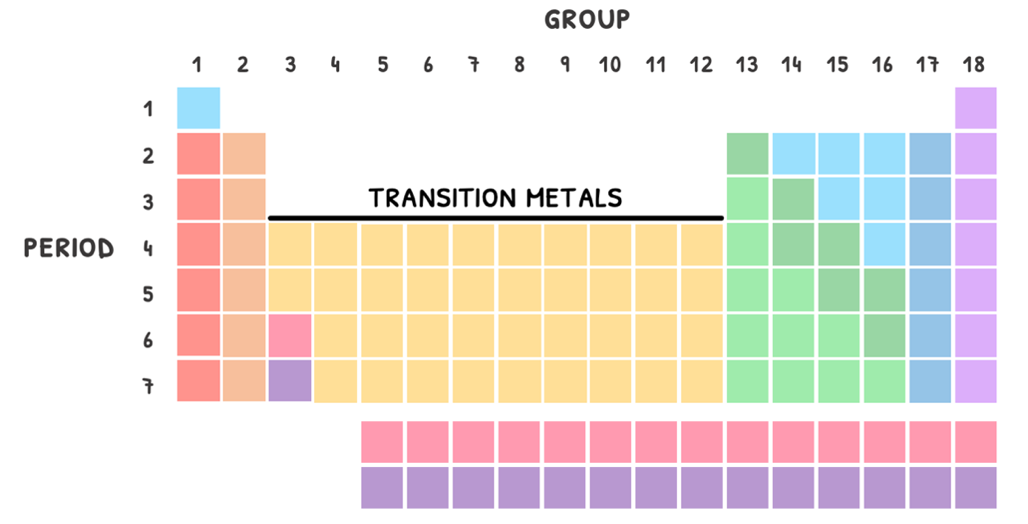
The d-block metals are found in groups 3-12. By definition, transition metals are elements that form ions with an incomplete d-orbital in one or more of its oxidation states. The exception to this is Zinc, which has a full d-orbital as an ion.
The common properties of transition metals include:
- Variable oxidation states
- Catalytic properties
- Magnetism
- Formation of complex ions
- Formation of colored compounds
Sail through the IB!
Sail through the IB!
Sail through the IB!
S3.2: Functional groups
Functional Groups
In Topic S3.2, you learn about organic chemistry, which is the study of organic molecules. These are molecules that contain carbon and form living organisms, and you need to able to identify and name organic molecules.
This starts by classifying them into different groups based off unique atom combinations, called functional groups. There are eight key functional groups to learn:
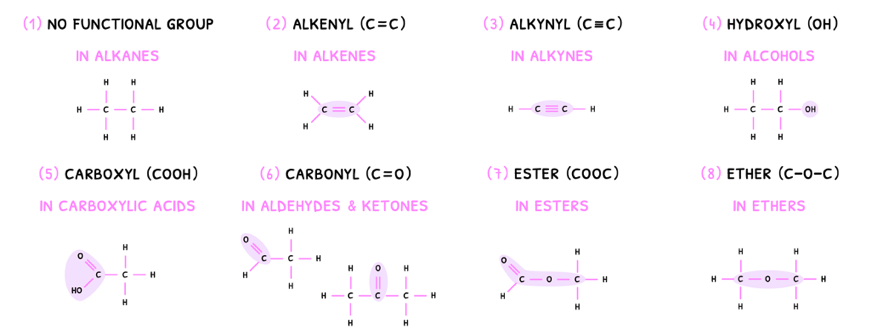
- No functional group - classed as an alkane.
- Alkenyl group - classed as an alkene.
- Alkynyl group - classed as an alkyne.
- Hydroxyl group - classed as an alcohol.
- Carboxyl group - classed as a carboxylic acid.
- Carbonyl group - classed as an aldehyde or ketone.
- Ester group - classed as an ester.
- Ether group - classed as an ether.
There are four additional groups that may appear, but you do not need to know the class:
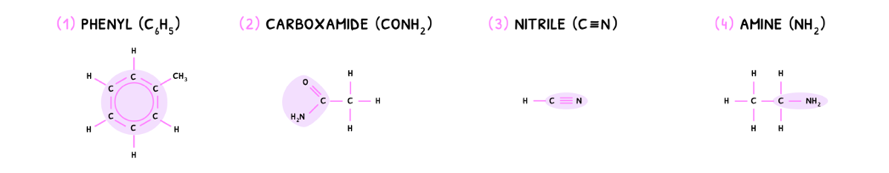
Homologous Series
Every single organic molecule will contain one or more of these functional groups. Molecules with the same functional groups but often different sizes are said to be part of a homologous series. This has four rules:
- All members contain the same functional group.
- All members can be defined by the same general formula.
- Each member differs from the adjacent member by a CH2 group.
- Each member exhibits similar chemical properties, and the series exhibits a gradation in physical properties.
For homologous series with one functional group, the general formulae you need to remember are:
- Alkanes have the general formula CnH2n+2.
- Alkenes have the general formula CnH2n.
- Alkynes have the general formula CnH2n-2.
- Alcohols have the general formula CnH2n+1OH.
- Carboxylic acids have the general formula CnH2n+1COOH.
For all homologous series, the general trend is that as molecule length increases, melting and boiling point increase due to an increase in London dispersion forces.
Sail through the IB!
Sail through the IB!
Sail through the IB!
S3.2: Further functional groups (HL)
Stereoisomerism
Remember that molecular isomers are species with the same molecular formula but a different structural formula. However, even with the same structural formula, isomers can exist due to the rotation of atoms in space. Thus, stereoisomers are two species with the same structural formula but different arrangements of atoms in space. These can be divided into two types:
- Conformational isomers
- Configurational isomers
Conformational isomers
Let's start with conformational isomers, which are created by the rotation of groups around a σ bond without breaking or reforming bonds. This can occur in both linear and cyclic compounds. To visualize this:
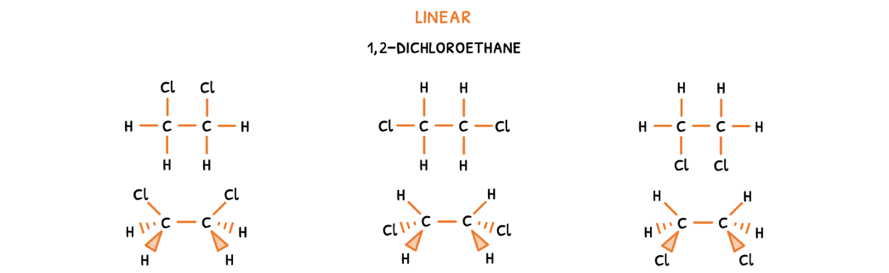
- Draw the possible 2D structural formulas by placing all groups in different positions on the carbons.
- Then, draw the molecular geometry of the molecule.
Here, it is visible that the groups are in different positions and can be rotated around the single bond to form each other.
To avoid drawing molecular geometries every time, the molecules are drawn head-on. Here, two isomers can be drawn:
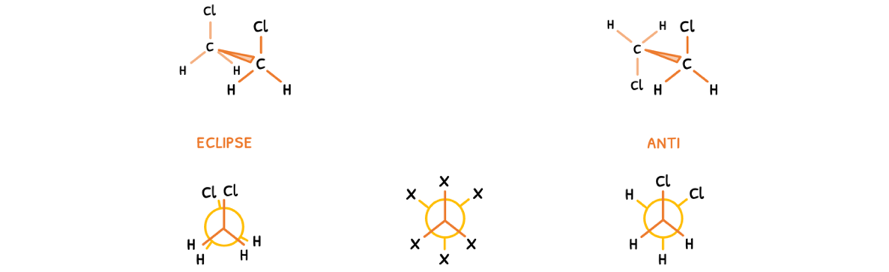
- Eclipse isomer - an isomer where the groups on either end are aligned and thus obscure one another.
- Anti isomer - an isomer where the groups on either are perfectly misaligned and thus all clearly visible.
In cyclic compounds, the conformational isomers are not dependent on rotation of the bonds, but the shape of the ring itself. Here, the same approach can be applied:
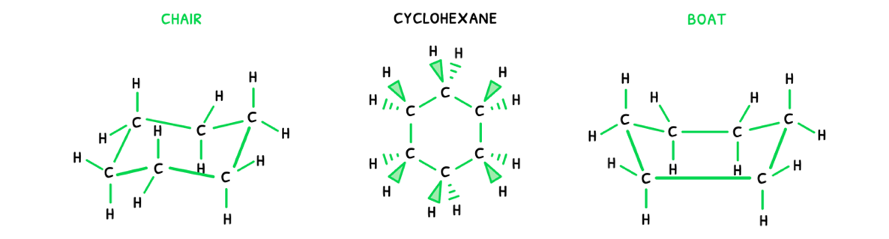
- Draw the possible 2D structural formula:
- Then, draw the molecular geometry of the molecule.
Here, it is visible that the ring can have two different shapes. This can be condensed into a simpler form:
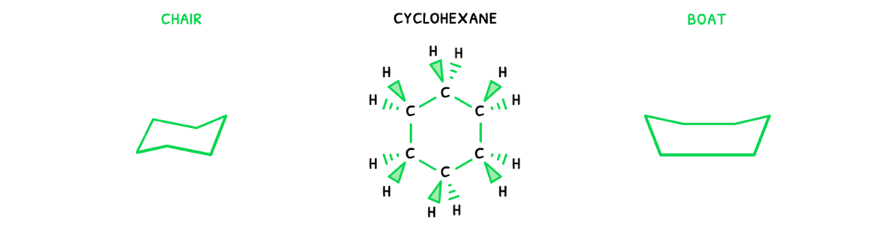
- Chair isomer - an isomer where the ring forms a chair shape.
- Anti isomer - an isomer where the ring forms a boat shape.