Ionization energy discontinuities
In the HL syllabus, you are supposed to appreciate that there are discontinuities in some of the periodic trends. The one example you need to understand this in is ionization energy across a period. What you learned is that:
- The number of protons increases, meaning the nuclear charge increases.
- However, there is no added energy level of non-valence electrons, maintaining the shielding effect.
- As a result, the effective nuclear charge increases across a group.
- Therefore, the electrostatic attraction between the nucleus and the valence electrons increases, and thus valence electrons require more energy to pull away.
- This ultimately means there is an increase in ionization energy, as shown below:
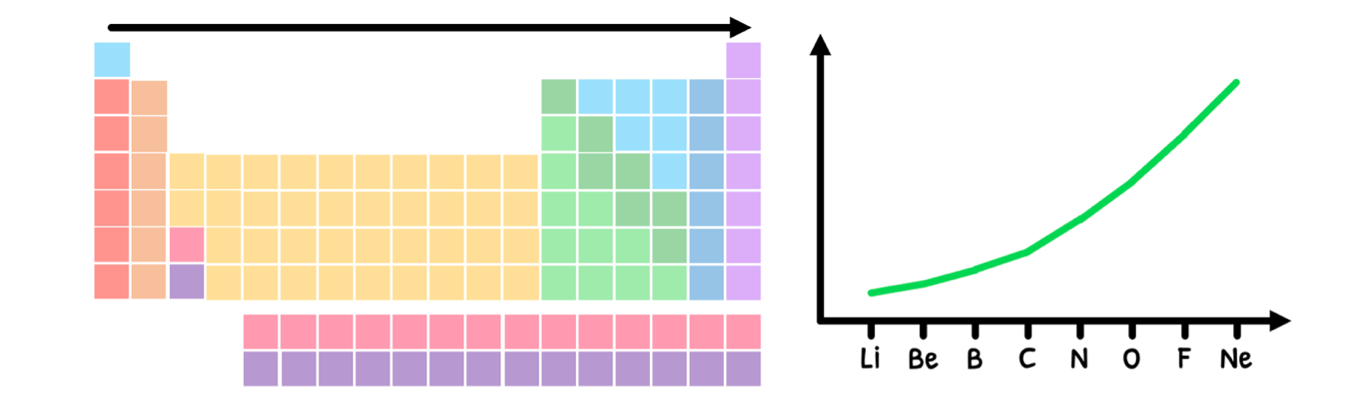
However, the trend is technically not as clean as this graph shows due to the added stability of half-full and full orbitals. Let's look at this in detail:
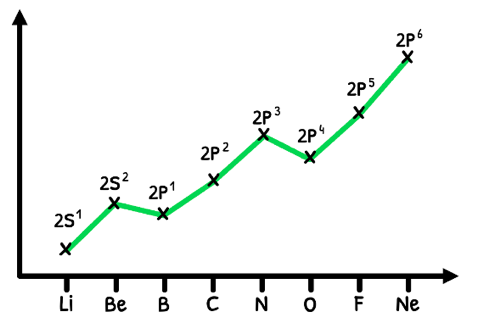
- The first exception, Boron, has the first electron in the 2p orbital. Despite an increase in effective nuclear charge, it is less stable than a full s-orbital so it has a lower ionization energy.
- The second exception, Oxygen, adds the fourth electron to the half-full p-orbital. Despite an increase in effective nuclear charge, the electron-electron repulsion causes instability, decreasing the ionization energy.
Transition metals
HL students also need to know the properties of the first row of transition metals.
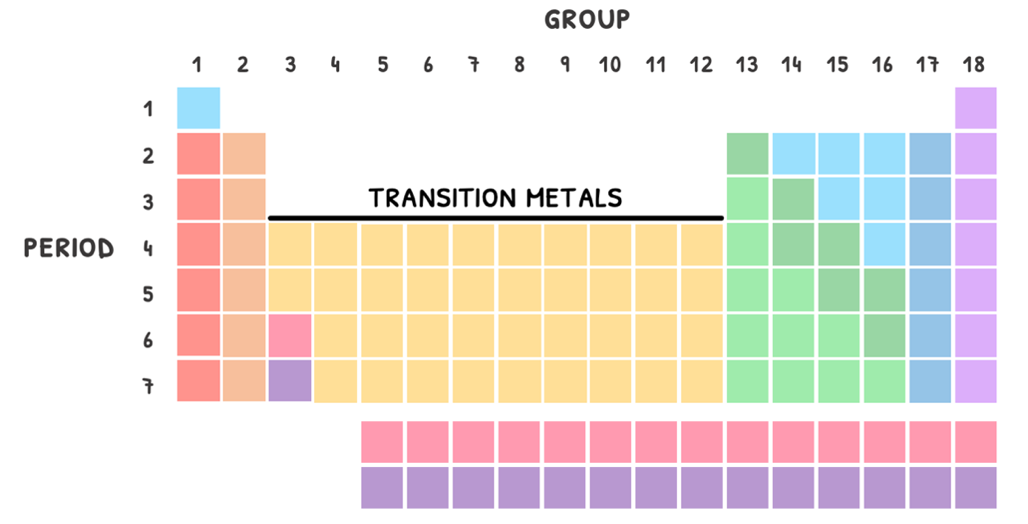
The d-block metals are found in groups 3-12. By definition, transition metals are elements that form ions with an incomplete d-orbital in one or more of its oxidation states. The exception to this is Zinc, which has a full d-orbital as an ion.
The common properties of transition metals include:
- Variable oxidation states
- Catalytic properties
- Magnetism
- Formation of complex ions
- Formation of colored compounds


