Functional Groups
In Topic S3.2, you learn about organic chemistry, which is the study of organic molecules. These are molecules that contain carbon and form living organisms, and you need to able to identify and name organic molecules.
This starts by classifying them into different groups based off unique atom combinations, called functional groups. There are eight key functional groups to learn:
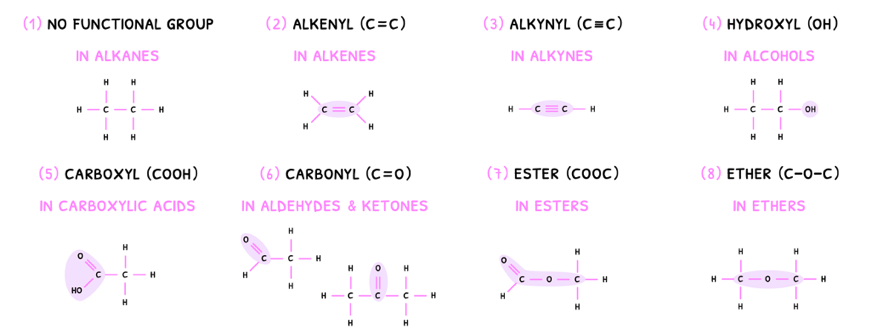
- No functional group - classed as an alkane.
- Alkenyl group - classed as an alkene.
- Alkynyl group - classed as an alkyne.
- Hydroxyl group - classed as an alcohol.
- Carboxyl group - classed as a carboxylic acid.
- Carbonyl group - classed as an aldehyde or ketone.
- Ester group - classed as an ester.
- Ether group - classed as an ether.
There are four additional groups that may appear, but you do not need to know the class:
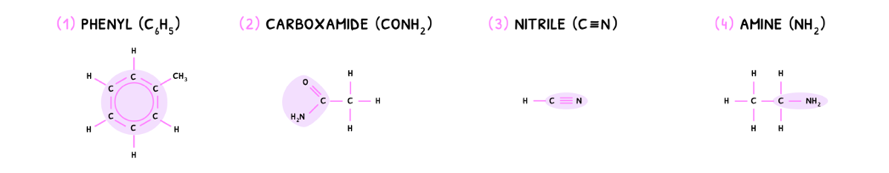
Homologous Series
Every single organic molecule will contain one or more of these functional groups. Molecules with the same functional groups but often different sizes are said to be part of a homologous series. This has four rules:
- All members contain the same functional group.
- All members can be defined by the same general formula.
- Each member differs from the adjacent member by a CH2 group.
- Each member exhibits similar chemical properties, and the series exhibits a gradation in physical properties.
For homologous series with one functional group, the general formulae you need to remember are:
- Alkanes have the general formula CnH2n+2.
- Alkenes have the general formula CnH2n.
- Alkynes have the general formula CnH2n-2.
- Alcohols have the general formula CnH2n+1OH.
- Carboxylic acids have the general formula CnH2n+1COOH.
For all homologous series, the general trend is that as molecule length increases, melting and boiling point increase due to an increase in London dispersion forces.

