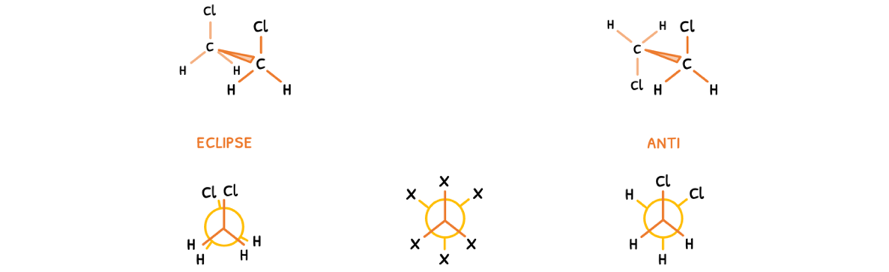Stereoisomerism
Remember that molecular isomers are species with the same molecular formula but a different structural formula. However, even with the same structural formula, isomers can exist due to the rotation of atoms in space. Thus, stereoisomers are two species with the same structural formula but different arrangements of atoms in space. These can be divided into two types:
- Conformational isomers
- Configurational isomers
Conformational isomers
Let's start with conformational isomers, which are created by the rotation of groups around a σ bond without breaking or reforming bonds. This can occur in both linear and cyclic compounds. To visualize this:
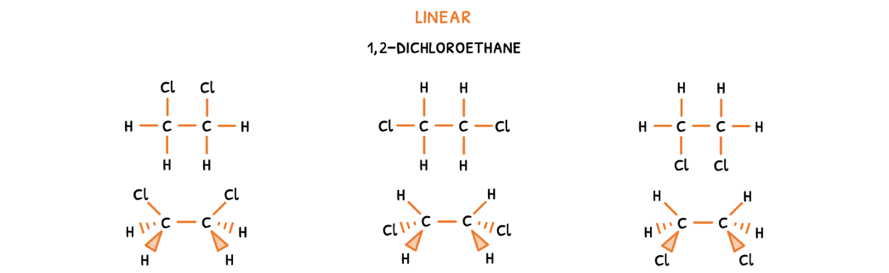
- Draw the possible 2D structural formulas by placing all groups in different positions on the carbons.
- Then, draw the molecular geometry of the molecule.
Here, it is visible that the groups are in different positions and can be rotated around the single bond to form each other.
To avoid drawing molecular geometries every time, the molecules are drawn head-on. Here, two isomers can be drawn:
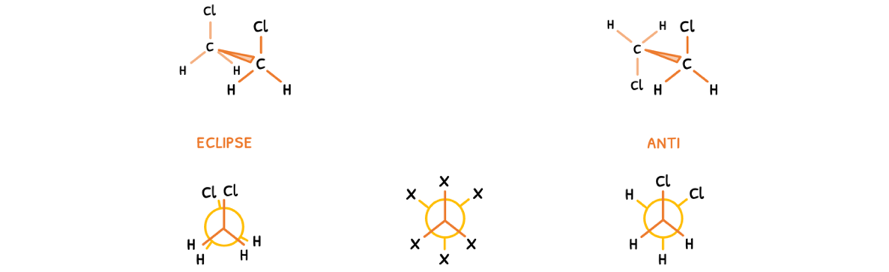
- Eclipse isomer - an isomer where the groups on either end are aligned and thus obscure one another.
- Anti isomer - an isomer where the groups on either are perfectly misaligned and thus all clearly visible.
In cyclic compounds, the conformational isomers are not dependent on rotation of the bonds, but the shape of the ring itself. Here, the same approach can be applied:
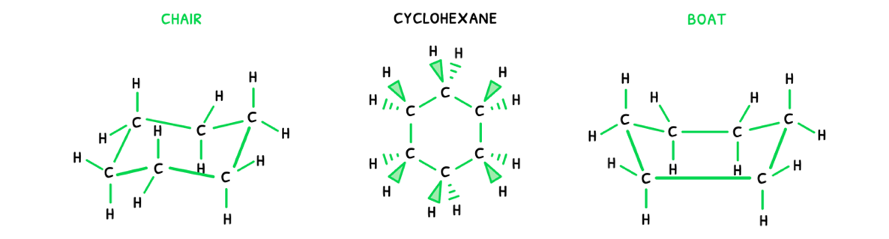
- Draw the possible 2D structural formula:
- Then, draw the molecular geometry of the molecule.
Here, it is visible that the ring can have two different shapes. This can be condensed into a simpler form:
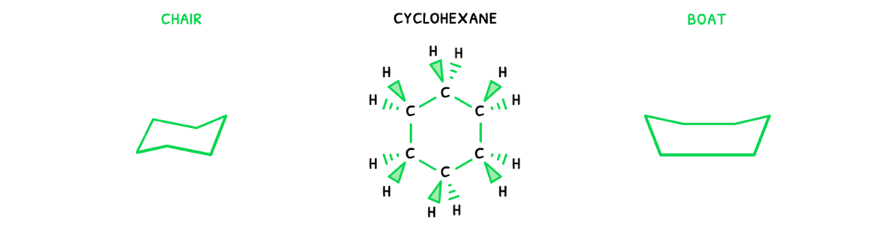
- Chair isomer - an isomer where the ring forms a chair shape.
- Anti isomer - an isomer where the ring forms a boat shape.