Hess's Law
In Topic R1.1, you learned about enthalpy change and how to measure it for exothermic reactions using a calorimeter. Whilst there are other ways to directly measure enthalpy changes, it is not always possible for every reaction. For this, another method is needed.
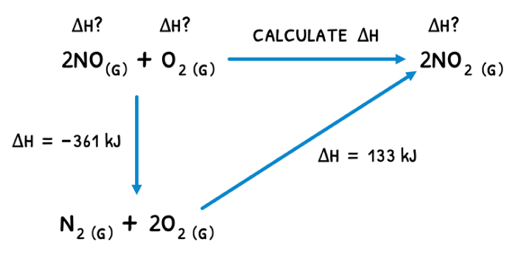
Hess's law describes that the enthalpy changes of a reaction is independent of the pathway between the initial and final states. This means that:
- From A to C, an enthalpy change of 10 kJ mol-1 may be observed.
- From A to B to C, the same end result was achieved and thus the overall enthalpy change is 10 kJ mol-1.
By breaking down a reaction into two sub-reactions, a reaction triangle can be formed and the unknown enthalpy change for the target reaction calculated.
Bond Enthalpies
Whilst Hess's law is an effective method to calculate enthalpy changes, it is also not always applicable. This leaves a last resort method - bond enthalpies.
Remember that energy is stored in chemical bonds. The bonds of interest are typically covalent bonds, which we know are impacted by bond length, bond strength, and bond polarity. The bond enthalpy is thus defined as the enthalpy change when one mole of covalent bonds is broken in a gaseous molecule. The formula for this is:
ΔH=ΔHbonds broken−ΔHbonds formed
In this context, the important concept to understand is the enthalpy change that occurs during bond formation and breaking.

- Bond formation releases energy. This thus has a -ΔH and is an exothermic reaction.
- Bond breaking requires energy. This thus has a +ΔH and is an endothermic reaction.
To remember which is which, use the phrase MExo BEndo (Making bonds is Exothermic, Breaking bonds is Endothermic).
Average Bond Enthalpy
However, as stated before, the bond enthalpy changes with bond length and bond strength. For example, moving from single to triple bonds will increase the amount of energy required to break the bond. Moving from single to triple bonds also decreases the bond length, hence a decrease in bond length increases the bond strength. This will all result in differences in the values of bond enthalpy.
The values in your data booklet are average bond enthalpies. These are calculated averages of bond enthalpies from a variety of gaseous compounds of which the specific bonds have been broken and the enthalpy measured. This means theoretical calculations using them will never exactly match literature measurements of enthalpy change, especially when using bond enthalpies for aqueous, liquid, or solid compounds.

