Spontaneity
In Topic R1, you learned more about enthalpy changes and in general, you have learned about how exothermic and endothermic reactions change the stability of chemical compounds. The next component you need to know is how this stability reflects the likelihood of a reaction occurring, termed spontaneity.
A spontaneous reaction is a reaction that occurs without energy input. However, both exothermic and endothermic reactions can be spontaneous even though endothermic reactions typically require an energy input. This is because spontaneity depends on three factors:
- Enthalpy
- Temperature
- Entropy
Entropy and Entropy Change
The last factor is new, so let's cover it. Entropy (S) is colloquially known as a measure of the disorder of energy. Officially, it is a measure of distribution of available energy among particles, measured in J K-1 mol-1.
Just as with enthalpy, entropy cannot directly be measured, but changes in entropy can. Therefore, the standard entropy change (ΔSø) is used for reactions. The formula for this is:
ΔS=ΔSproducts−ΔSreactants
Whilst it is easy to understand how enthalpy changes can be determined, it is less obvious with entropy. A way to think of entropy is that systems with a greater distribution of energy have a higher disorder and thus a higher entropy.
This can easily be seen as substances progress from solids to liquids to gases. As a solid, the particles are in very organized structure and as a gas, the particles have no organized structure and randomly move.
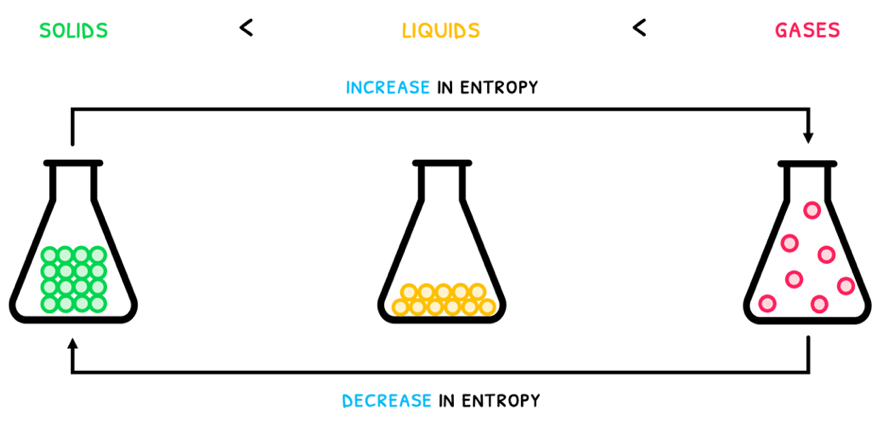
Thus, from solids to gases, entropy increases and so the entropy change is positive. From gases down to to solids, entropy decreases and so the entropy change is negative.

