Heat
In Topic S1.1, you learned that bond formation and breaking requires or releases energy. In this topic, you learn how to understand why this occurs and calculate the associated energy changes, particularly in relation to the heating of substances.
Remember that temperature is the average kinetic energy of particles. From this, you can appreciate that when fast particles from hot objects react with slow particles from cold objects, they exchange energy. Heat (Q) is thus the thermal energy transferred between two objects with different temperatures.
Since the faster particles tend to give up energy during collisions, heat naturally flows from hot objects to cold objects, or high temperatures to low temperatures. During this:
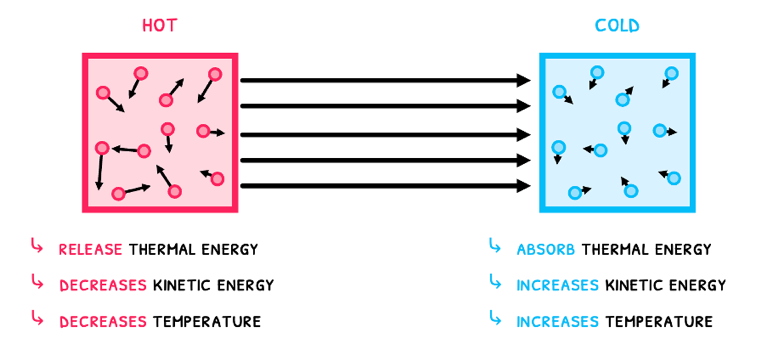
- Hot objects release thermal energy, which decreases the kinetic energy of their particles. As a result, their temperature decreases.
- Cold objects absorb thermal energy, which increases the kinetic energy of their particles. As a result, their temperature increases.
Enthalpy and Enthalpy Change
However, the kinetic energy of a particle is not the only energy it possesses. It also contains chemical energy in the form of intramolecular and intermolecular bonds. The total internal energy of the particle is thus the sum of the kinetic and chemical energy of particles, termed the enthalpy (H).
However, a particle's enthalpy cannot be measured, so we instead calculate the enthalpy change (ΔH) in kJmol-1, which is equal to the heat transfer.
As a result, two types of enthalpy changes are possible: negative and positive. This is dependent on the type of reaction:
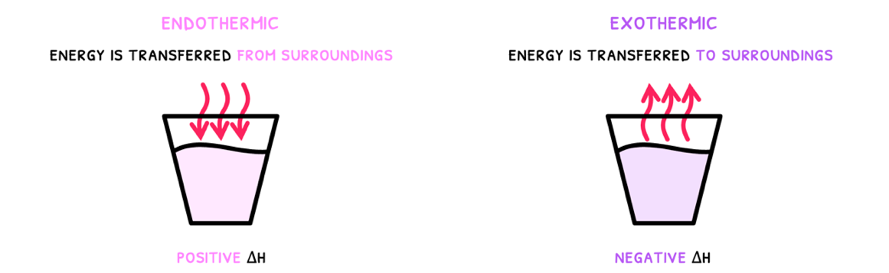
- Exothermic - a reaction that transfers energy to the surroundings, releasing it. This has a -ΔH and a decrease in temperature. The products of this reaction are thus lower in energy and more stable.
- Endothermic - a reaction that transfers energy from the surroundings, absorbing it. This has a +ΔH and an increase in temperature. The products of this reaction are thus higher in energy and more unstable.


