Higher Level Covalent Bonding
In the HL syllabus of Topic S2.2, you need to understand several more components of covalent bonding, including:
- Two additional VSEPR domain geometries
- Using formal charge for Lewis structures
- Electron sharing in bonds
- Hybridization
VSEPR Theory
Starting with VSEPR, you need to know the additional two domains:
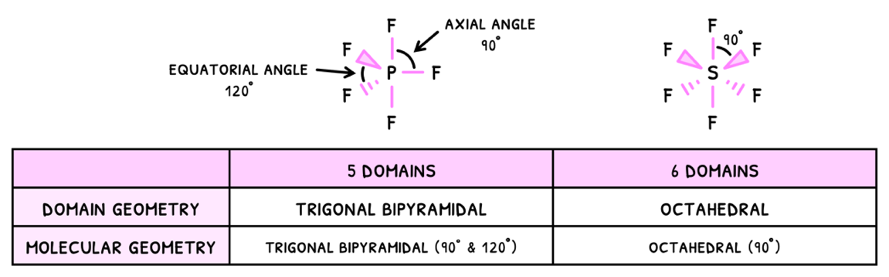
- Trigonal bipyramidal - 5 electron domains
- Octahedral - 6 electron domains
Just as before, the presence of a lone pair will decrease the bond angles by 2.5°, and this effect compounds. However, in some cases the pairs cancel one another and so the bond angles do not decrease.
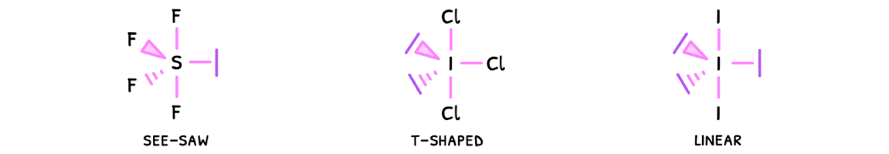
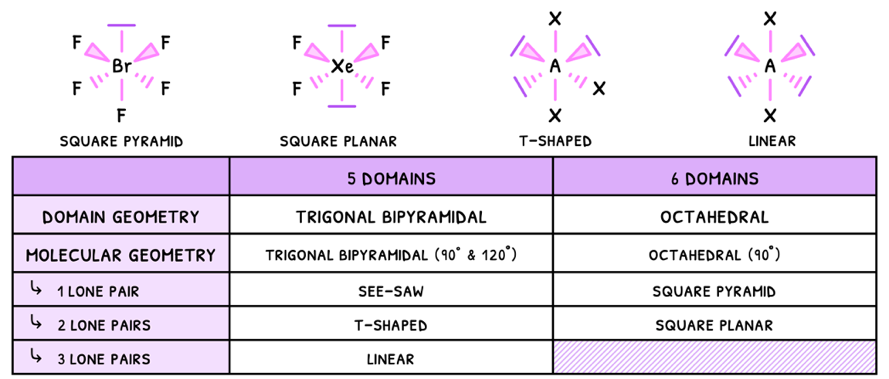
- Linear - electrons are removed from the equatorial plane, so the angle here becomes 180°.
- Square planar - electrons are removed from the axial plane, so the angles become 90°.
Formal Charge
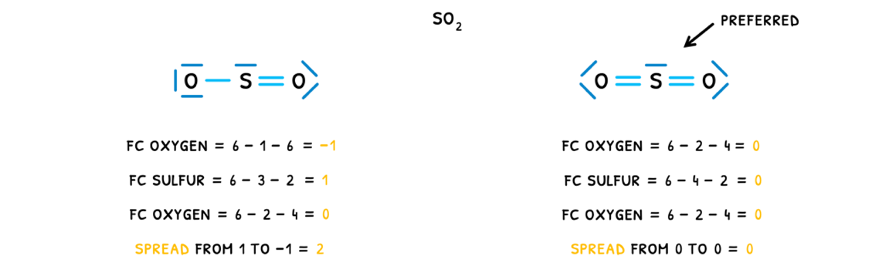
Next, you need to know more details about resonance structures. This is because when drawing Lewis structures, you will trouble finding the correct one when resonance structures exist. However, the concept of formal charge is incredibly useful to confirm you have chosen the correct Lewis structure.
Formal charge is used to decide between different Lewis structures because the Lewis structure with the smallest formal charge is preferred. To do this:
- Calculate formal charge for each atom in the compound. The formula for this is:
valence electrons−21bonding electrons−non bonding electrons
- Summate all the formal charges.
Performing this for two possible Lewis structures of SO2, the preferred structure can be found: