Metallic Bonds
This topic focuses on the last intramolecular bond - the metallic bond. These are the weakest type and occur only between metals. It is defined as the electrostatic attraction between a regular lattice of positive ions and a sea of delocalized electrons.
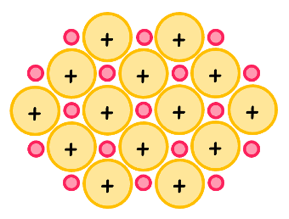
When considering metallic substances, there are two factors that affect the strength of the metallic bond:
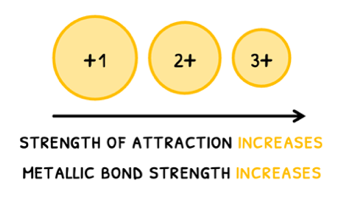
- Ionic charge - as charge increases, the strength of the attraction between the positive ions and delocalized electrons increases. Therefore, metallic bond strength increases and melting and boiling point increase.
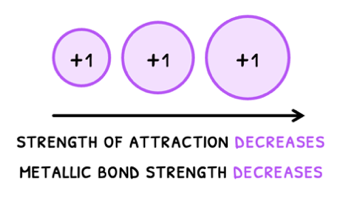
- Ionic radius - as ionic radius increases, the attraction decreases as the nucleus of the positive ion is further from the delocalized electrons. Therefore, metallic bond strength decreases and melting and boiling point decrease.
It is important to note that charge has a greater effect on attraction than size!
Interestingly, this association between bond strength and melting point only applies up to Period 3, after which atomic arrangement starts to affect melting point as well. For instance, Mercury is liquid at room temperature!
Properties of Metals
However, in general all metals have similar properties:
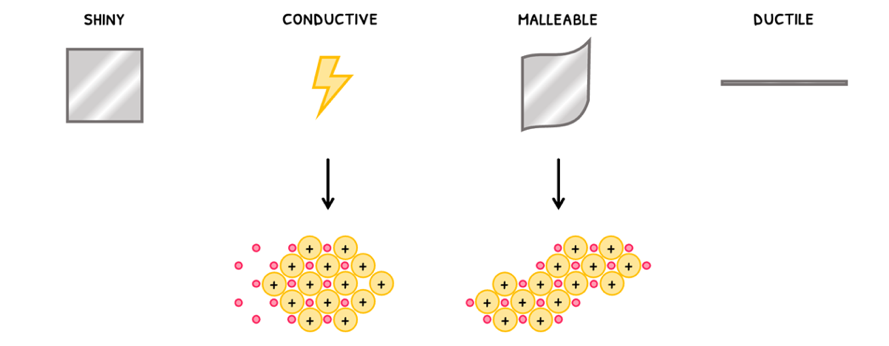
- They are shiny.
- They are conductive. Remember this is because the sea of electrons can move and carry charge.
- They are malleable. Since electrons attract the positive ions in all directions, the layers can slide over one another.
- Finally, metals are ductile, meaning they can be stretched out and made into a wire.



