Introduction to Bonding
In this topic, you will explore the types of bonds within molecules, called intramolecular bonds (aka. interatomic bonds), and the bonds between molecules, called intermolecular bonds (aka. intermolecular forces).
There are three intramolecular bond types you need to be aware of. These are shown below in order of decreasing strength:
- Covalent bonding (strongest)
- Ionic bonding
- Metallic bonding (weakest)
When considering covalent compounds (formed from covalent intramolecular bonds), there are also three intermolecular bonds/forces you need to be aware of. These are shown below in order of decreasing strength:
- Hydrogen bonds (strongest)
- Dipole-dipole forces
- London dispersion forces (weakest)
You may have heard ionic bonds are stronger than covalent, and whilst there are certain situations in which this can be the case, it depends on the environment in which the ionic species is found (solvated, vacuum, etc.) and the IB does not recognise this. Therefore, for your IB exam you should focus on the intramolecular ranking listed above.
Ionic bonding
An ionic bond is the electrostatic attraction between oppositely charged ions. Remember that ions are formed when atoms lose or gain electrons to become cations or anions.
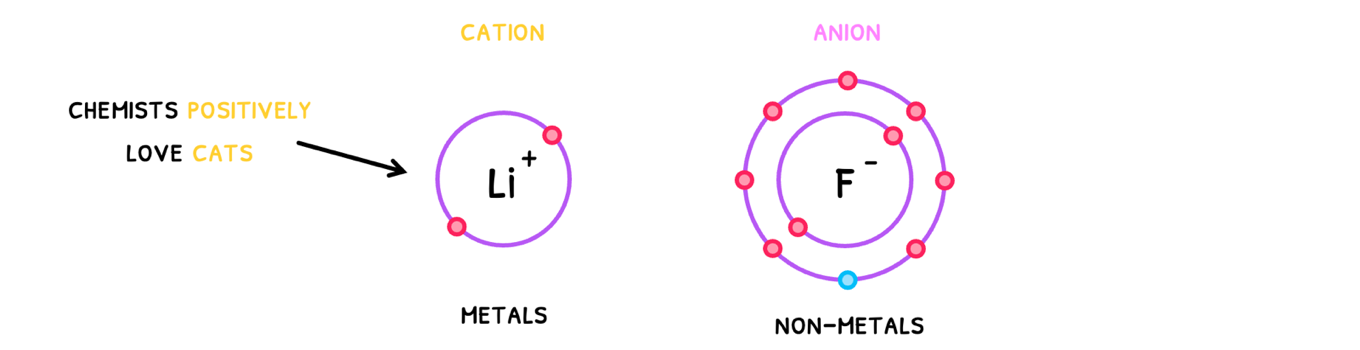
- Cations - ions with a positive charge.
- Anions - ions with a negative charge.
To remember which is which, use the phrase "Chemists positively love cats".
Note that cations are typically metals and anions are typically non-metals. Thus, to know if they have an ionic bond, look at the electronegativity difference of the elements. If it is greater than 1.8, they form an ionic bond!
Additionally, note that when atoms become ions, they become isoelectric with another atom or ion. This means that they have the same electron configuration!
Ionic Compounds and Properties
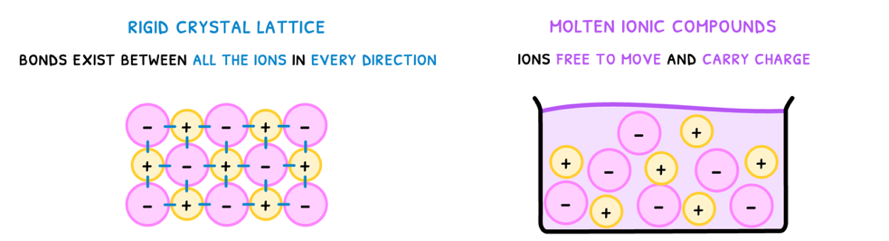
Next, you need to understand the structure that is formed when many ionic bonds come together as an ionic compound. However, the structure depends on the state of the compound:
- Solid - forms a rigid crystal lattice as bonds exist between all ions in every direction.
- Liquid - no lattice as ions are free to move.
Knowing the structure of ionic compounds, you can understand their properties:
- High melting and boiling point - due to the strong electrostatic attraction between the ions in their respective lattices, ionic compounds require large inputs of energy to break apart these forces.
- Volatility - this refers to the ease at which a substance vaporizes. Due to the strong electrostatic attraction, ionic compounds have very low volatility.
- Solubility - ionic compounds dissolve in polar solvents but not in non-polar solvents. Only solvents which contain molecules with partial charges can dissolve ionic compounds. These partial charges can pull individual ions from the lattice.
- Conductivity - ionic compounds cannot conduct electricity in the solid state as the ions are fixed in place. However, when molten, the ions can move around freely and thus conduct electricity.

