Bonding Triangles
Classifying bonding as either ionic, covalent, or metallic can be limiting and does not allow us to classify compounds by their specific properties when they do not fit perfectly within a category.
Many materials are composite e.g carbon fibre or concrete, meaning that they are mixtures of two different compounds in different phases and the individual compounds retain their own properties. The understanding of the mixture of properties is necessary for the identification of materials for specific purposes.
The bonding triangle, sometimes called the Arkel-Ketelaar triangle, represents bonding as a continuum and illustrates differences between ionic, covalent, or metallic bonding as well as everything in between. It is useful to reiterate that usually, compounds with a higher electronegativity difference have a more ionic character and compounds with a low electronegativity difference have a covalent character.
The bonding triangle is split into fields being metallic, ionic, covalent or polar covalent, and is set up with a y and x-axis, where the x-axis represents the average electronegativity, and the y-axis represents the difference in electronegativity. In the bonding triangle the three angles are the following bonding characteristics:
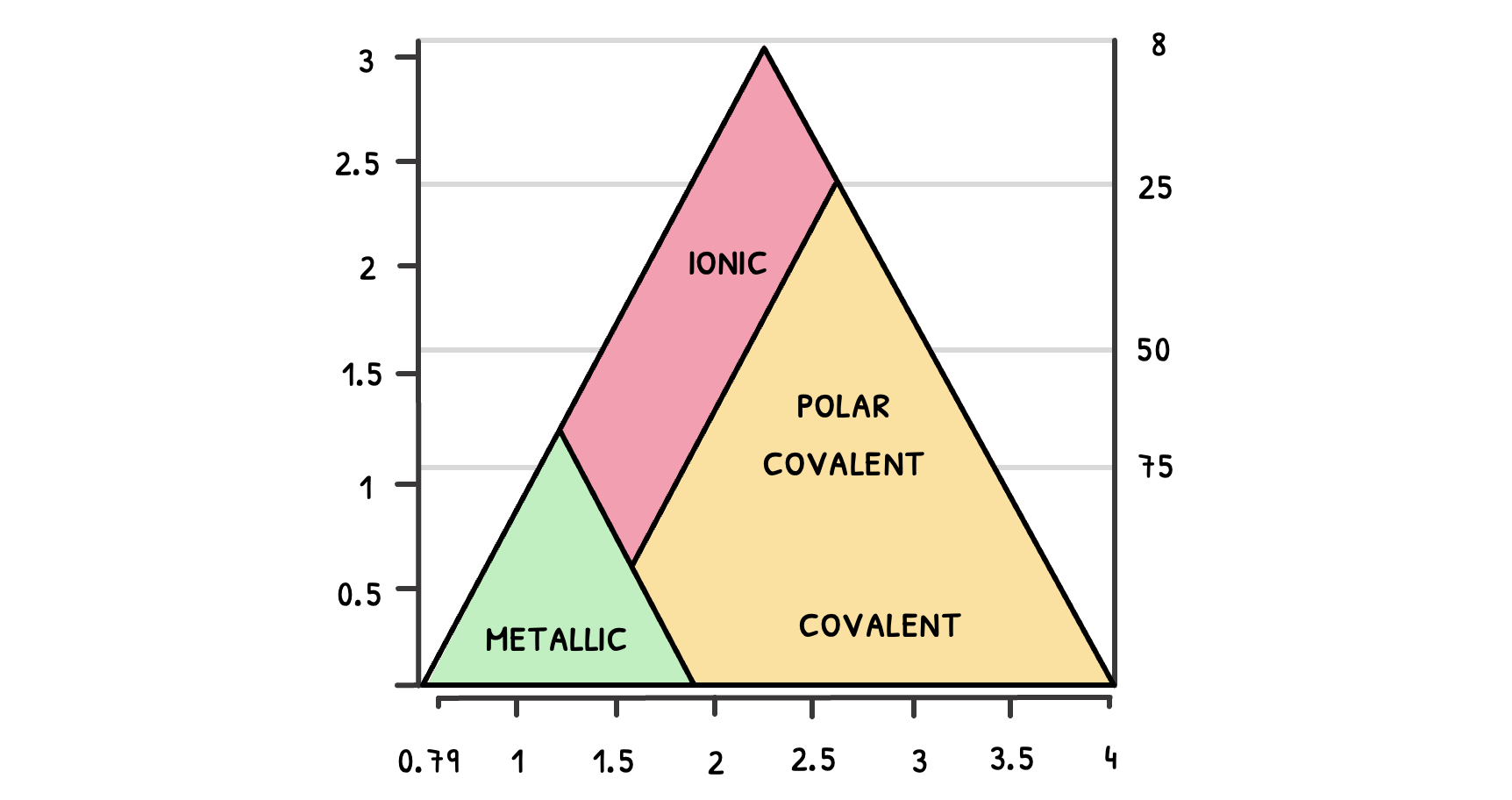
- Strongly ionic on the top
- Strongly covalent on the bottom right
- Strongly metallic on the bottom left
The most metallic atom with the lowest electronegativity is Caesium, the most electronegative atom is Fluorine and hence the most ionic molecule is Caesium fluoride, which could represent the 3 angles of the bonding triangle. The bonding triangle can be used to work out the properties of a variety of compounds. In SL you will only have to work out properties of binary compounds.
Alloys
However, metals can bond with one another, completely changing their properties. When this happens, they are termed alloys.
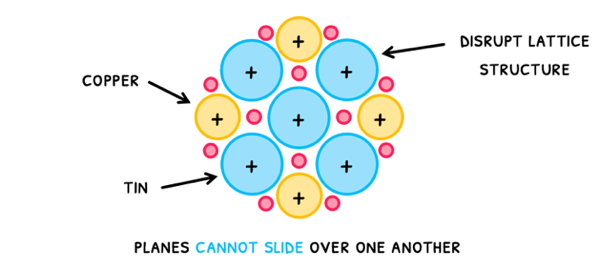
Alloys tend to be stronger and stiffer than their individual elements, because the two elements have differently sized positive ions. This disrupts the lattice structure, preventing the planes from sliding over one another.
Common alloys include:
- Steel = Iron (Fe) + Carbon (C)
- Stainless steel = Iron (Fe) + Carbon (C) + Chromium (Cr)
- Bronze = Copper (Cu) + Tin (Sn)
- Brass = Copper (Cu) + Zinc (Zn)
- Pewter = Tin (Sn) + Antimony (Sb)

