Electromagnetic waves
Whilst you know about electrons and their orbitals in Topic S1.2, you also need to understand the discovery electron energy levels and transitions between them. This is evidenced by the absorption and release of energy by electrons in the form of an electromagnetic wave.
Electromagnetic waves are also known as electromagnetic radiation, which are a form of energy that travel as a wave. Remember that the basic components of waves are velocity (c), wavelength (λ), and frequency (f).
Whilst all electromagnetic radiation has a velocity of 3 x 108 ms-1, wavelength (λ), and frequency (f) change to give different wave types and energies. These are all described by the electromagnetic spectrum, wherein:
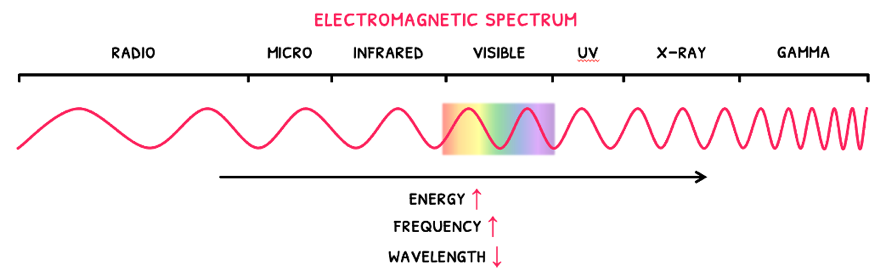
- As wavelength decreases, frequency increases.
- As wavelength decreases, energy increases.
Since the electromagnetic spectrum shows all the wave types, it is known as a continuous line spectrum. This is more specifically defined as a spectrum that shows all possible wavelengths. On the contrary, a line spectrum only shows particular wavelengths.
Absorption & emission spectra
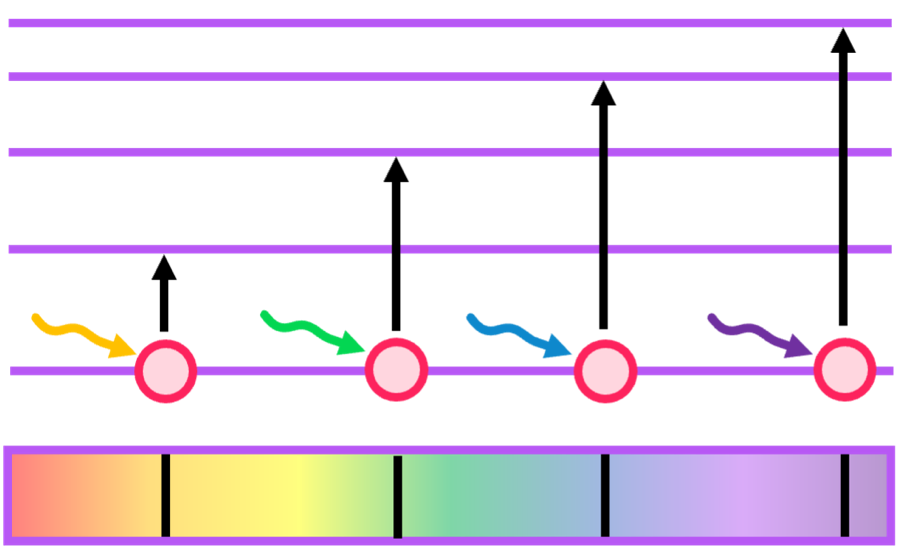
Relating this to electron energy transitions, when light shines on an element and is then turned off, two spectra are formed: absorption and emission.
- Absorption spectra - electrons will absorb specific wavelengths that allow them to promote to another energy level. These absorbed wavelengths are thus not detected, as shown below.
- Emission spectra - electrons will re-emit the wavelengths that they absorbed to demote to their base energy level. These emited wavelengths are thus detected.
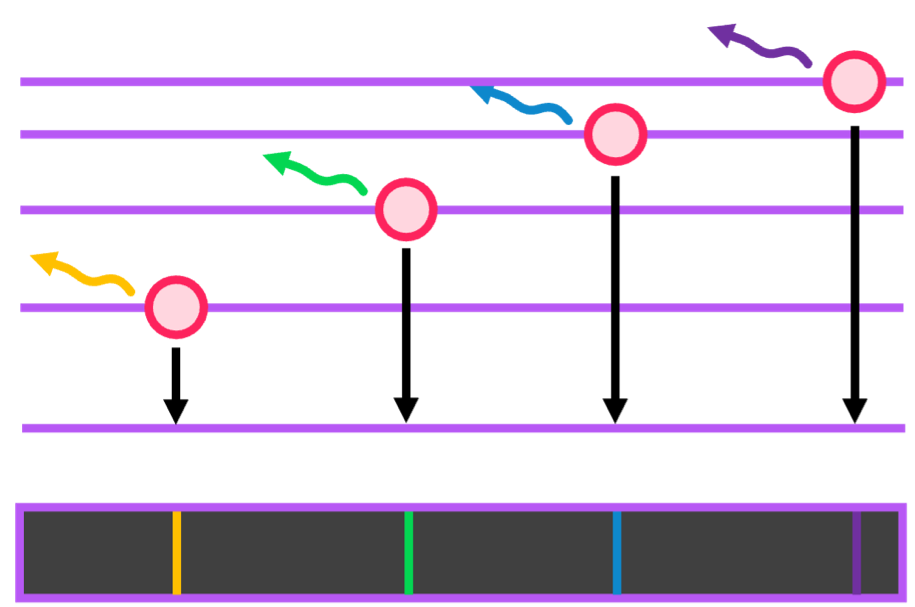
The emission spectrum is an example of a line spectrum, and if you put both the emission and absorption spectra together, the continuous spectrum is reformed.


