Calculating energy changes
In Topic S1.3, HL students need to be able to quantify the energy changes electrons undergo when they absorb or emit electromagnetic waves. Remember that from the electromagnetic spectrum that wave energy is proportional to its frequency (f). The formula for this is:
E=hf
In this, h is the Planck constant and equal to 6.63 x 10-34 Js. An alternative formula can be formed by substituting in the wavespeed equation.
c=fλ
λc=f
E=λhc
Ionization energy and trends
An additional concept you are expected to understand is that of ionization energy. Remember that if enough energy is supplied to an electron, it can escape the nucleus's grasp and go beyond the convergence limit, creating an ion.
This is the first ionization energy, defined the energy required to remove one electron from a mole of gaseous atoms, measured in kJ mol-1. Any subsequent ionizations have the terms second ionization energy, third ionization energy ... et cetera. The reaction can be described via the following chemical equation:
X(g) → X+(g) + e-
It is important to understand that only one of the electrons in the outermost shell, termed the valence shell, is removed from the atom, and that the energy required to achieve this is completely dependen on the charge attractions with the nucleus.
In Topic S3.1, common trends in the periodic table will be discussed. However, more detail is required for HL students when discussing ionization energy.
For this, and future trends, a few terms are commonly used so must first be defined:
- Electrostatic attraction - the electrical attraction of opposite charges between the positive nucleus and the negative electrons.
- Nuclear charge - the degree of attraction by protons in the nucleus to electrons.
- Shielding effect - the phenomenon that non-valence electrons block the nuclear charge from reaching the valence electrons.
- Effective nuclear charge - the actual nuclear charge that reaches the valence electrons and attracts them after the shielding effect.
Whilst you will use these terms explain trends in Topic S3.1, in this topic you are expected to understand the trend in successive ionization energies. These are defined as the energies required to remove consecutive electrons from a mole of gaseous atoms, measured in kJ mol-1.
You are required to be able to explain this for any element, but the trend is based on the electronic configuration and is thus the same for any element.
To generalize this to any element, remember the following rules:
- For consecutive ionizations, ionization energy will increase due to an increase in effective nuclear charge.
- For ionizations from full s-orbitals, ionization energy will have a large increase due to the stability of a full s-orbital.
- For ionization from full p-orbitals, ionization will have an even larger increase due to a drop in energy level and shielding, significantly increasing effective nuclear charge and ionization energy.
When viewing magnesium's ionization energy graph, all these rules are in effect.
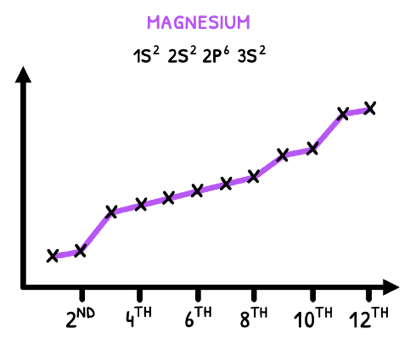
- Ionization energy increases from start to end.
- There is a larger increase in the 9th ionization as this takes the first electron from the 2s orbital.
- There is an even larger increase in the 3rd and 11th ionization energies as these remove the first 2p and 1p electrons, respectively.
