The mole
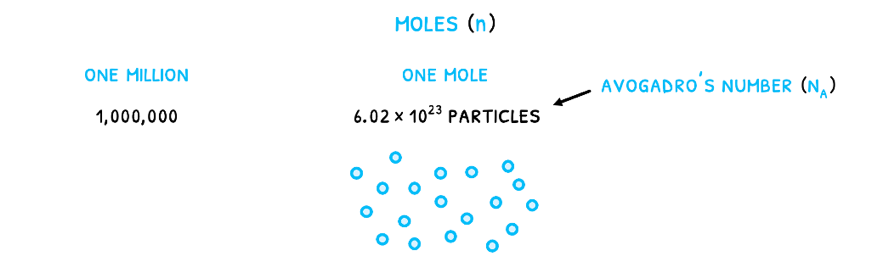
In reactions, substances are often measured in moles (n). This is a measure of the number of particles given by Avogadro’s number (NA) equal to 6.022 x 1023 particles/mole. It relates to the number of particles that would make Carbon-12 exactly 12 grams as the standard element. Therefore, just like one million is 1,000,000, one mole is 6.022 x 1023 particles.
Note that this describes the number of particles so 1 mole of any diatomic molecule, such as H2, has 1.2044 x 1024 atoms because every molecule (and therefore particle) has two component atoms.
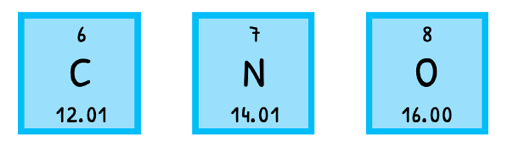
The mass of an atom can be expressed as molar mass (M), the mass of one mole of a substance in gmol-1. The most often used one is the relative atomic mass (Ar), the weighted mean molar mass of all naturally occurring isotopes of the element relative to Carbon-12. This is the case due to Avogadro's number.
Calculating molar mass is possible with the equation:
molar mass (M)=moles (n)mass (m)
Formula Mass
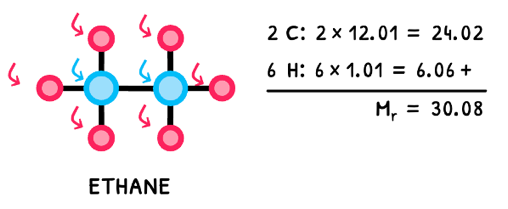
The relative atomic mass of each element is then used in further calculations. These often involve calculating the molar mass of a molecule. This is referred to as the relative formula mass (Mr), is the sum of the relative atomic masses of its components. For example, the relative atomic mass of hydrogen is 1.01 gmol-1 and of carbon is 12.01 gmol-1 so the relative formula mass of C2H6 is 30.08 gmol-1, as shown below!
Formulas
Lastly, when forming molecules and compounds, it is important to understand how to correctly communicate them. This can be done via three forms of formulas: molecular, empirical, or structural.
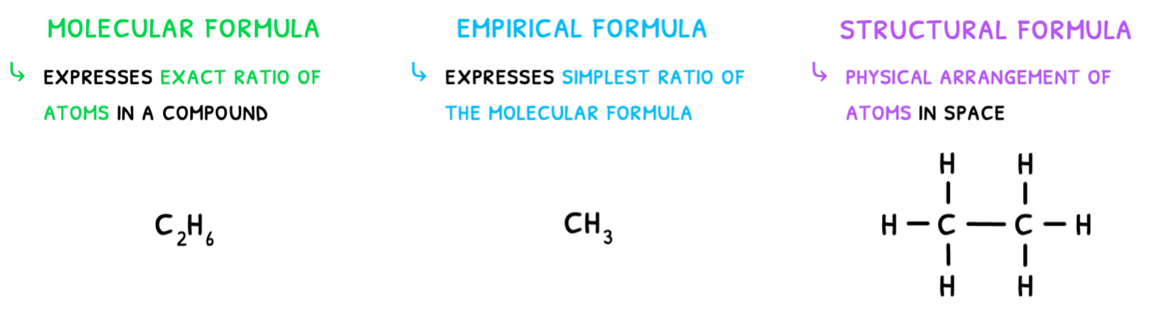
- Molecular formulae state the compound's atomic composition in the exact ratio they appear. For ethane, this is C2H6.
- Empirical formulae state the compound's atomic composition in the most simplified ratio. For ethane, the ratio is simplified to CH3.
- Structural formulae show the compound's atomic composition and the physical arrangment of the atoms in space.



