Ideal Gases
To complete your understanding Topic S1, the IB expects you to further understand the behavior of gases. Since different gases will behave differently in certain conditions, the concept of an ideal gas has been created to predict their behavior. An ideal gas has the following properties:
- No forces between particles.
- Perfectly elastic collisions.
- Particles have no volume.
- One mole occupies 22.7 dm3 at STP or 24.0 dm3 at RTP.
Note that real gases will only behave like ideal gases at moderate tohigh temperatures, low density, and low pressures.
The ideal gas equation thus relates the main properties of gases: pressure, volume, moles, and temperature. The formula for this is:
PV=nRT
In this formula, the gas constant (R) is 8.314 JK-1mol-1, whilst pressure is in kPa, volume is in dm3 and temperature in K. However, most of the time you will find that moles is kept constant pressure, volume, or temperature are changed. This changes the equation to:
T1P1V1=T2P2V2
Ideal Gas Laws
Together, pressure, volume, and temperature are thus considered the main factors of gases. Their effects on gas behavior are described by the ideal gas laws. These are as follows:
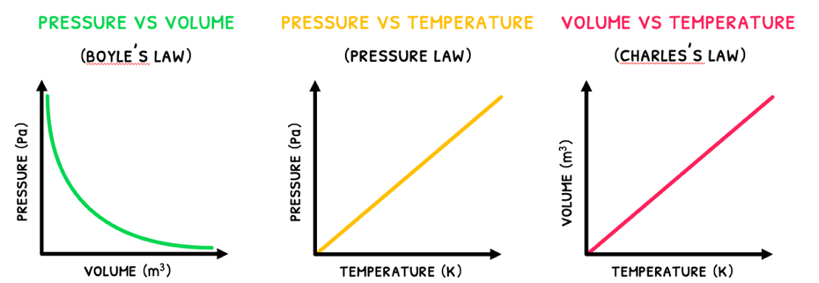
- Boyle's law - this states that the relationship between pressure and volume is inversely proportional when temperature stays constant. Gases exert a higher pressure when contained at smaller volumes due to an increased frequency of collisions with the container.
- Guy-Lussac's law - this states that the relationship between pressure and absolute temperature is directly proportional when volume stays constant. When temperature reaches absolute zero (0 K or -273°C) both kinetic energy and pressure is equal to 0.
- Charles's law - this states that the relationship between volume and temperature is directly proportional when pressure stays constant. Gases have more kinetic energy at higher temperatures and will collide with more frequency and energy. This requires more volume to fulfill the requirement of constant pressure.
