Electron-pair sharing
The last type of reaction you need to know about involves the sharing of electron pairs. During these reactions, two terms frequently come up:
- Electrophile - this is an electron-deficient reagent with a positive or partially positive charge that accepts electron pairs.
- Nucleophile - this is an electron-rich reagent with a negative or partially negative charge that donates electron pairs.
These will result in two different types of reactions occurring:
- An electrophile will accept an electron pair as a part of electrophilic substitution or electrophilic addition.
- A nucleophile will donate an electron pair as a part of nucleophilic substitution.
Electrophilic Addition
Let's begin with electrophilic addition. This is the process by which alkenes reacts with halogens or hydrogen halides to form halogenoalkanes, or water to form alcohols.
Alkenes are the species typically involved because they contain an alkenyl (C=C) function group and have the general formula CnH2n.
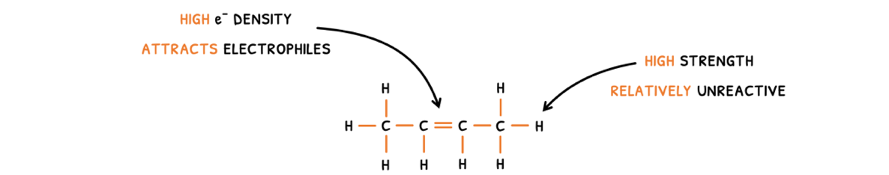
Although the C-C and C-H bonds make alkenes unreactive, the C=C is an area of high electron density that attracts electrophiles.
So, during electrophilic addition:
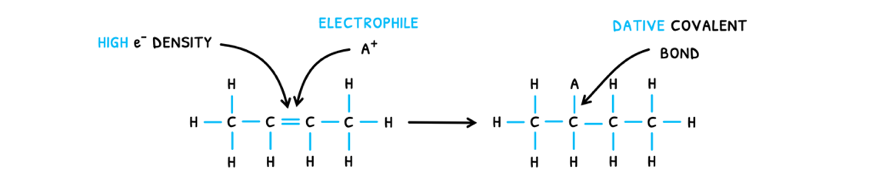
- The high electron density of the C=C bond attracts electrophiles.
- The C=C bond breaks to donate an electron to the electrophile, forming a dative covalent bond.
- This forms a positive carbocation, attracting a nucleophile.
- The nucleophile donates an electron to the carbocation, forming a dative covalent bond.
The products differ depending on the electrophile:
- Diatomic halogen - C2H4 + Br2 → C2H4Br2
- Halogen halide - C2H4 + HBr → C2H5Br
- Water - C2H4 + H2O → C2H5OH
Nucleophilic Substitution
Second, nucleophilic substitution. Once a dihalogenoalkane or halogenoalkane is formed, it can swap nucleophiles. This involves the attack of a halogenoalkane by a nucleophile, swapping the halogenoalkane for the nucleophile. The nucleophile is typically OH- to produce an alcohol.
SL students are not expected to be able to explain this process in detail, but need a basic overview of what happens. During nucleophilic substitution:
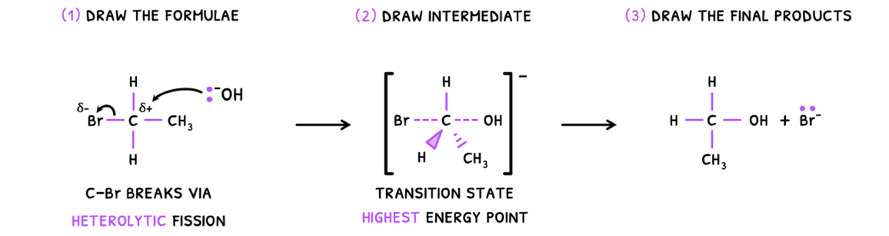
- The C-X bond is polar, forming a dipole. This causes two events:
- A more reactive nucleophile (OH-) attacks the positive dipole by donating a pair of electrons.
- This forces the C-X bond to break via heterolytic fission, donating both electrons to the halogen.
- Depending on the mechanism, this may or may not form an intermediate transition state.
- Once the breaking and formation is complete, an alcohol and halogen ion are formed.


