Electron transfer
The second type of reaction you need to know about is electron transfer. This is typically subdivided into the oxidation and reduction of species, which can translate to the production of electrical energy. Within this, it is first important to understand what oxidation and reduction mean:
The old definitions state that:
- Oxidation is the process by which a species undergoes a loss of hydrogen or gain of oxygen.
- Reduction is the process by which a species undergoes a gain of hydrogen or loss of oxygen.
However, sometimes species neither gain nor lose hydrogen or oxygen, making it difficult to tell which processes is occurring. This led to the new definitions, which state that:
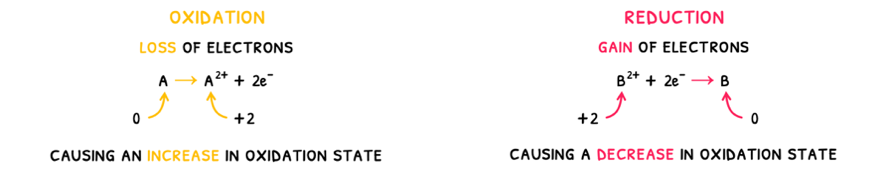
- Oxidation is the process by which a species undergoes a loss of electrons.
- Reduction is the process by which a species undergoes a gain of electrons.
A mnemonic that can be used to remember these definitions is OIL RIG: Oxidation Is Loss of electrons and Reduction Is Gain of electrons.
Oxidation States
However, when two compounds swap ligands, it is sometimes difficult to tell whether a species is losing or gaining electrons. Here, you need to remember the conecpt of oxidation state, as this can be revisited.
- Oxidation is the process by which a species undergoes a loss of electrons, causing an increase in oxidation state.
- Reduction is the process by which a species undergoes a gain of electrons, causing a decrease in oxidation state.
Thus, by observing how the oxidation state of an element changes, you can tell whether it oxidizes or reduces.
Redox Reactions
Now, naturally if in a reaction a species oxidizes, another species reduces. This can also occur within the same species. Example of both of these scenarios are shown below.
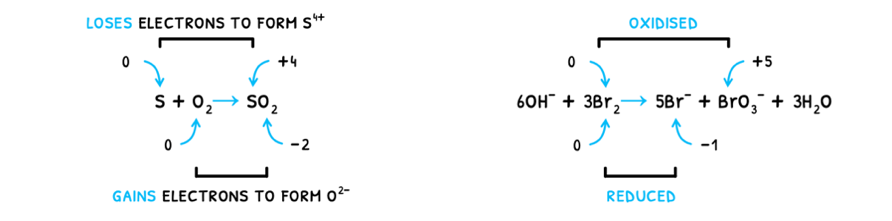
These types of reactions are called redox reactions, as both oxidation and reduction occur. Within this, there are two species:
- Oxidizing agent - the species that becomes reduced, and thus causes the oxidation of another species.
- Reducing agent - the species that becomes oxidized, and thus causes the reduction of another species.

