Calculations for Acids and Bases
Whilst you learned the basics of acids and bases in the SL syllabus, you need to be able to perform calculations involving them in the HL syllabus.
For this, a handful of extra concepts need to be learned, including:
- pOH
- pKa
- Ka
- pKb
- Kb
Calculating pOH
Let's start with pOH. Whilst pH is "power of hydrogen" to describe acidity, pOH is the "power of hydroxide" to describe alkilinity. Thus, it is simply a measure of OH- concentration. The formula for this is:
[OH−]=10−pOH
Taking the log of both sides, a more common formula appears. The formula for this is:
pOH=−log10[OH−]
Naturally, it is also possible to plot pOH as a log scale of OH- concentration. This runs in the opposite direction of pH from 14 to 0. However, a 10-fold change in OH- concentration still causes a change of 1 pOH.
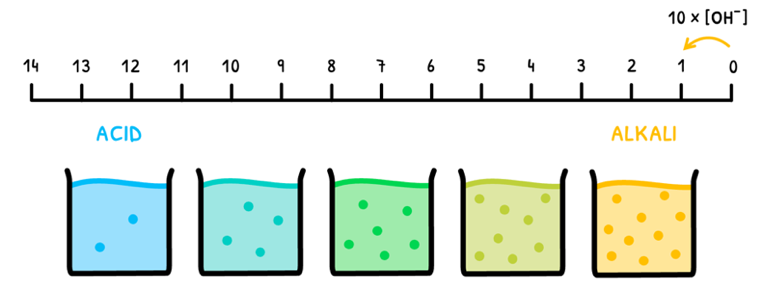
The scale can again be divided into distinct sections;
- A pOH of 14 indicates that [OH-] = 10-14 mol dm-3, meaning the solution is strongly acidic.
- A pOH of 7 indicates that [OH-] = 10-7 mol dm-3, meaning the solution is neutral.
- A pOH of 0 indicates that [OH-] = 1 mol dm-3, meaning the solution is strongly alkaline.
Calculating pKw
Since pH is based off of Kw, it follows that pOH is too. Let's run through the math. Remember that:
Kw=[H+][OH−]
Just like pH and pOH, we can establish a "power of water" by taking its log so that:
pKw=−log10Kw
Applying this to the concentrations of H+ and OH- too, we get:
pKw=−log10[H+][OH−]
pKw=pH+pOH
At 298 K, it is important to understand that for any solution pKw = 14 (since Kw = 10-14). Thus:
pH+pOH=14
