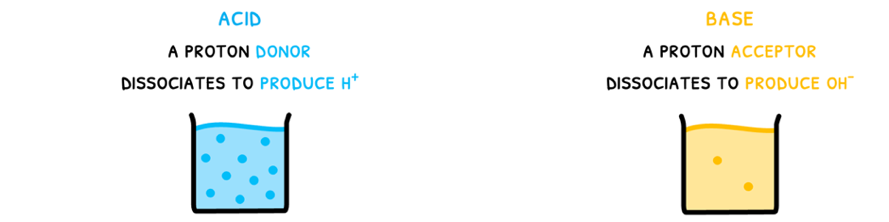Proton transfer
Topic R3.1 focuses on proton transfer reactions, the most common of which are reactions involving acids and bases. However, their properties are rather complex and require an in-depth look to understand the full process. Acids and bases can be described by three theories:
- Ionic theory - involves the formation of protons.
- Brønsted-Lowry theory - involves the transfer of protons.
- Lewis theory - involves the transfer of electrons, so covered in Topic R3.4.
Ionic Theory
Let's start with ionic theory, which is very simplistic. It has two key principles:
- Acids dissolve in water to produce H+ at a concentration greater than 1.0 x 10-7 mol dm-3.
- Bases dissolve in water and can neutralize acids.
Brønsted-Lowry Theory
Brønsted-Lowry theory is more complicated. This also has two principles:
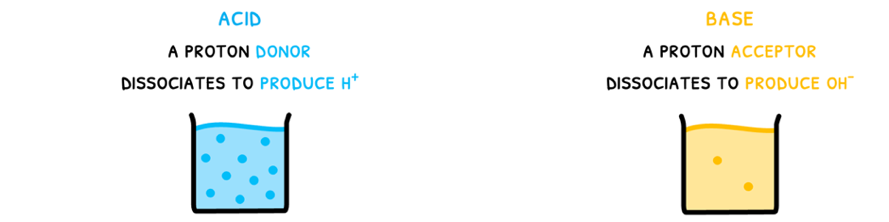
- Acids dissociate to produce H+, which is subsequently donated to another molecule.
- Bases dissociate to produce OH-, and subsequently accepts a proton.
Conjugate Acids and Bases
The species formed from these reactions are:
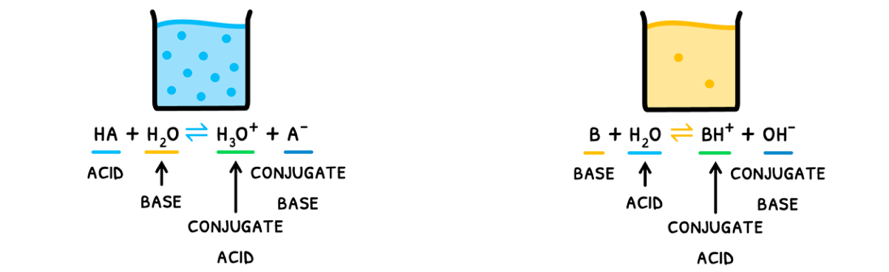
- Conjugate base - the ion formed by an acid donating a proton. As a result, this ion can now accept a proton and is thus a base.
- Conjugate acid - the ion formed by a base accepting a proton. As a result, this ion can now donate a proton and is thus an acid.
As you can see, a conjugate acid-base pair thus differs by one proton.
However, some species can act as both both Brønsted-Lowry acids and bases, which are called amphiprotic species. An example is HCO3-:
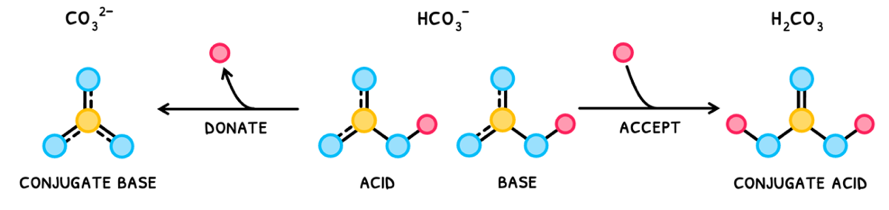
- It can donate a proton to become CO32-.
- It can accept a proton to become H2CO3.