Osmolarity
In Topic A1.1, you learned about the properties of water and that it dissolves polar substances via hydrogen bonds or dipole-dipole forces. B2.1, you learned about osmosis and aquaporins. Here, you need to learn about a key practical in biology involving potato cores in different solutions of water and observing the effects of osmosis. However, to effectively understand the practical, a few terms need to be defined first.
- Osmoles – this is the moles of solute particles that are dissolved in a solution.
- Osmolarity – this is a solution’s concentration expressed as the osmoles per liter. Sometimes you may see the term Osmolality, which is osmoles per kilogram, so remember the distinction!
With these definitions, two solutions can be compared using three terms:
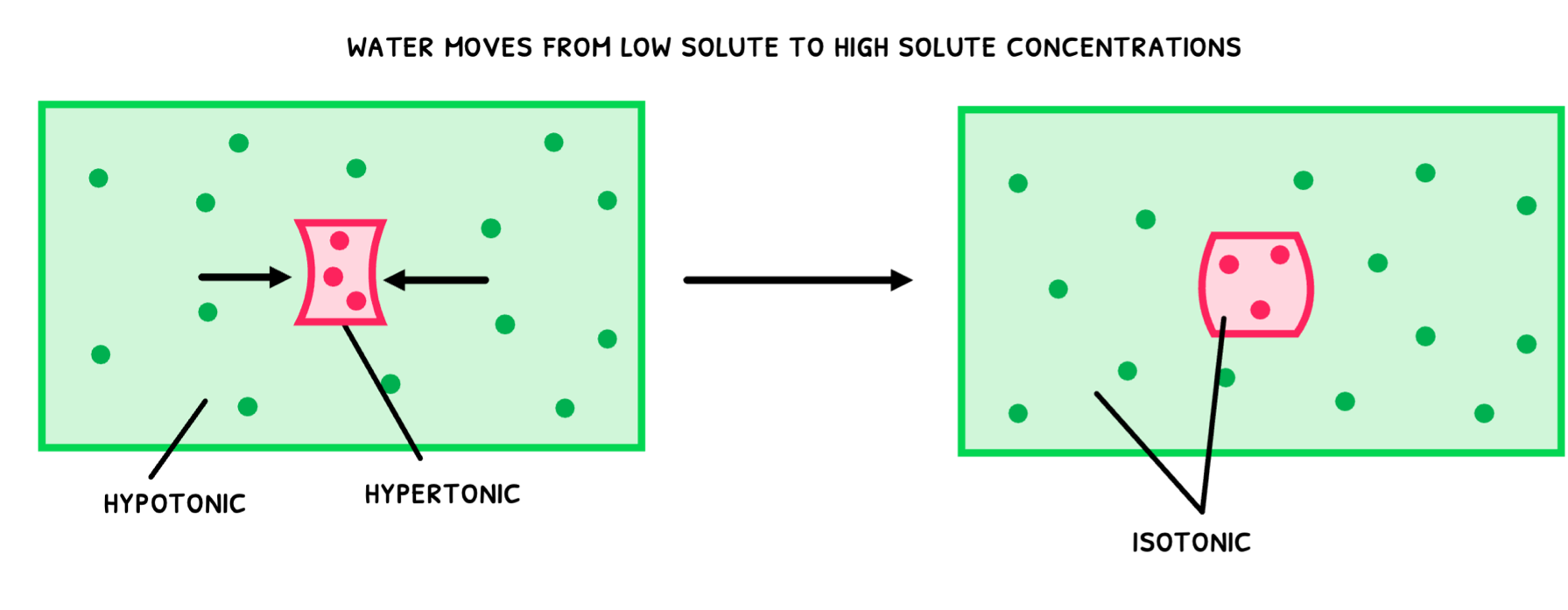
- Hypertonic – this refers to a solution with a higher osmolarity than another solution.
- Hypotonic – this refers to a solution with a lower osmolarity than another solution.
- Isotonic – this refers to two solutions with equal osmolarity.
Applying these to the potato, three situations can arise:
- A potato is placed in a hypotonic solution. Remember that this means that the solution’s solute concentration is lower than the potato’s. As a result, water moves from the low solute concentration (the solution) to the high solute concentration (potato) via osmosis. This water movement into the potato causes it to swell.
- A potato is placed in a hypertonic solution. Remember that this means that the solution’s solute concentration is higher than the potato’s. As a result, water moves from the high solute concentration (the potato) to the low solute concentration (the solution) via osmosis. This water movement out of the potato causes it to shrink.
- A potato is placed in an isotonic solution. Remember that this means that the solution’s solute concentration is the same as the potato’s. As a result, water does not move, and the potato does not change in size.
Osmosis in plant cells
Since a potato is a plant, we have a cell wall to consider in these scenarios. It causes a phenomenon called turgidity, which is the pressure exerted on the cell wall. This pressure is also called the turgor pressure and it is important to the structural rigidity of a plant:
- In a normal plant cell, the vacuole is filled with water, exerting turgor pressure on the cell wall. This fills the cell wall and helps it remain rigid, providing structure to the plant.
- If plant cell loses water, its turgor pressure is reduced and eventually lost, causing the plant to wilt. Plasmolysis refers to the complete loss of turgidity in a plant cell and is irreversible.
- If the plant gains more water than normal, its turgor pressure is too high. Normally, this would cause the cell to swell, but this is mostly counteracted by the cell wall, resulting in minor swelling. In this state, the cell is said to be turgid.
