IB Biology Sub-topic B1.1 Notes
Atoms and bonding
This topic predominantly focuses on the living processes of cells and organisms in terms of the chemical reactions involved, called molecular biology. For this, it is important to understand what molecules are and how they are held together. To provide a complete understanding, let’s start with atoms.
Atoms are the most basic unit of an element, composed of protons and neutrons in a nucleus, surrounded by orbiting electrons, as shown below.
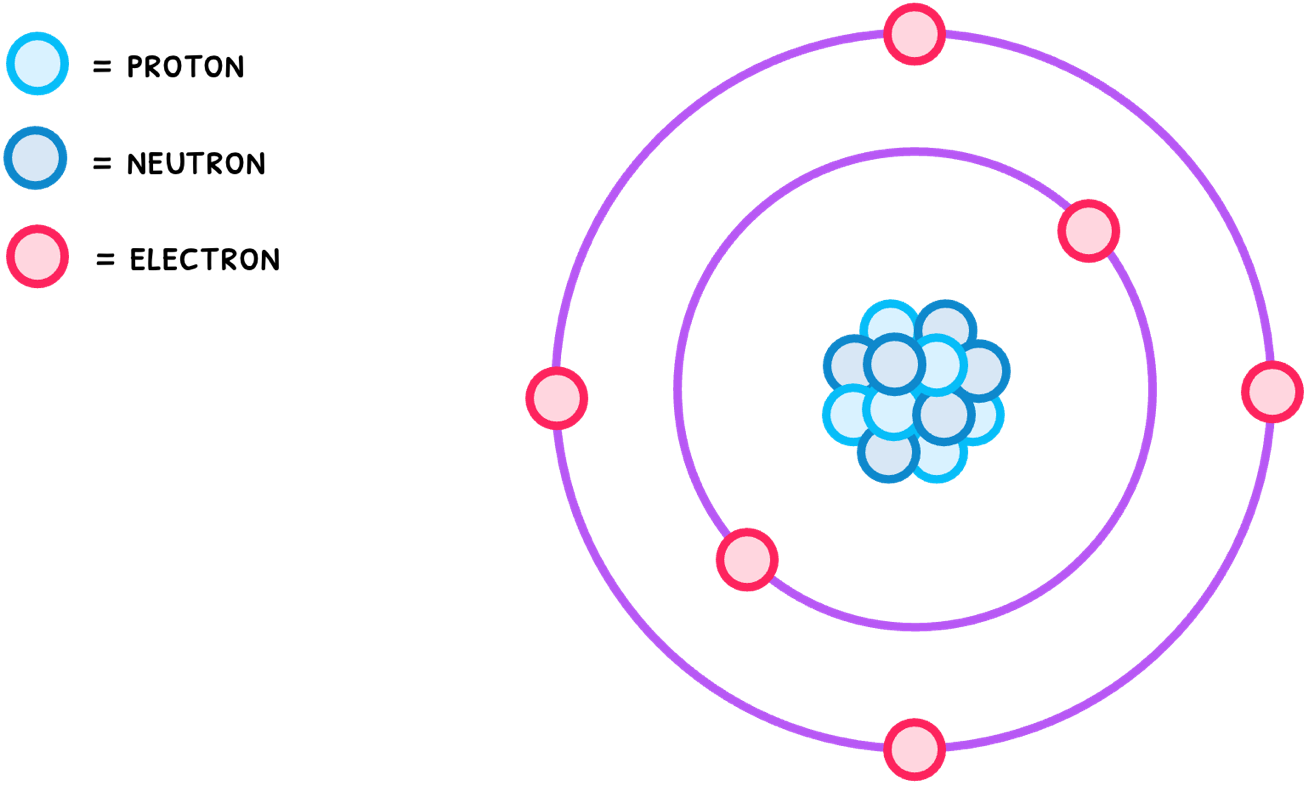
When two atoms come close together, the nucleus of one atom attracts the electrons of the other and vice versa. This ends up meaning that the two nuclei will share electrons between themselves, forming what is called a covalent bond. There are three types of covalent bonds:
- Single covalent bond – each atom shares one electron, placing two electrons in the bond.
- Double covalent bond – each atom shares two electrons, placing four electrons in the bond.
- Triple covalent bond – each atom shares three electrons, placing six electrons in the bond.
Polar and non-polar bonds
Molecules are thus the complexes that form when two or more atoms are held together by covalent bonds. Ultimately, how the electrons are shared determines what type of molecule is formed. Electrons can be shared in two ways: equally or unequally.
- The electrons can be shared equally, meaning both nuclei have the same strength and so the electrons lie in the middle of the bond.
- The electrons can be shared unequally, meaning one nucleus is stronger and so pulls the electrons in the bond closer to itself.
As a result of this electron sharing, two types of molecules are formed: polar or non-polar.
- Polar molecules are formed when there is unequal sharing, so one side of the molecule has more electrons than the other. As a result, the molecule has a negatively charged pole and a positively charged pole, called a dipole.
- Non-polar molecules are formed when there is equal sharing, so all sides of the molecule have the same number of electrons and no poles form.
Macromolecules and monomers
Now that you understand how molecules are formed and whether they are polar or non-polar, you need to understand that organic molecules are categorized into two types:
- Monomers - these are the simplest units of organic molecule types. Examples include:
- Monosaccharides - carbohydrate monomers.
- Amino acids - protein monomers.
- Nucleotides - DNA and RNA monomers.
- Macromolecules - these are monomers linked together to form larger molecular structures. Two monomers linked together are called a dimer, and three or more monomers linked together are called a polymer. Examples include:
- Polysaccharides - carbohydrate macromolecules
- Polypeptides - protein macromolecules
- Nucleic acids - nucleic acid macromolecules