Metabolism
In Topic C1.1, you focus on metabolism. An organism’s metabolism is defined as the complex network of interdependent and interacting chemical reactions occurring in living organisms. These chemical reactions can be divided into two types:
- Anabolic reactions – these are reactions that combine monomers to form macromolecules. As a rule of thumb, any anabolic reaction you see in IB Biology will be a condensation reaction. Remember that this produces a macromolecule and water.
- Catabolic reactions – these are reactions that break down macromolecules into monomers. As a rule of thumb, any catabolic reaction you see in IB Biology will be a hydrolysis reaction. Remember that this uses water to split a macromolecule.
Enzymes
Both anabolic and catabolic reactions are catalyzed by enzymes. They are defined as proteins that speed up the rate of chemical reactions by lowering their activation energy. Let’s explore this:
- Every chemical reaction requires an energy input to occur, called the activation energy. This can be thought of as a spark required to light a fire.
- This spark is usually provided when particles collide into one another during their random continual motion.
- When the resulting collision energy is higher than the activation energy of the reaction, then the “spark catches” and the reaction occurs. However, if the collision energy is lower than the activation energy, then the reaction does not occur.
Enzyme collisions
Enzymes are thus important to control metabolism and the rate at which it occurs. They do this by colliding with substrates at a binding region called the active site. This is composed of a few amino acids forming a 3D structure and requires substrates that have:
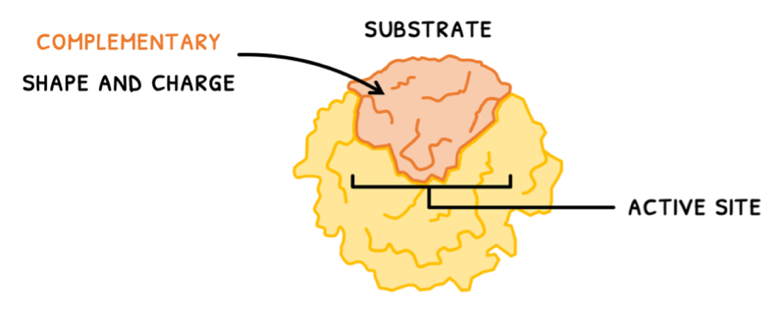
- A complementary shape and charge
- The correct orientation
- Sufficient collision energy
