R-groups
Whilst you now understand the basic structure of proteins and how they form polypeptides via peptide bonds, you know need to understand how these form 3D structures to become mature proteins. This all begins with R-groups, which is the only chemically variable component of the amino acid.
It is this variable group that provides the different amino acids with a host of different properties:
- Hydrophobic R-groups - in soluble proteins, folded into the protein’s interior. In insoluble proteins, present on the protein's exterior.
- Hydrophilic R-groups - in soluble proteins are arranged on the exterior of the protein
- Polar R-groups - participate in dipole-dipole bonding and hydrogen bonding.
- Charged R groups - create electrostatic attractions or repulsions which will influence the ultimate structure, stability and function of the protein.
Some R-groups are pH sensitive and accept or donate protons in accordance with the surrounding pH. This variation in R-groups and its accompanying effect on all potentially different chemical properties facilitates proteins versatility in a host of different functions, to include catalytic, transport, building and signal transduction.
Protein structure
Once a polypeptide chain has been formed, it can fold to form the protein. You are expected to be familiar with the four levels of protein structure:
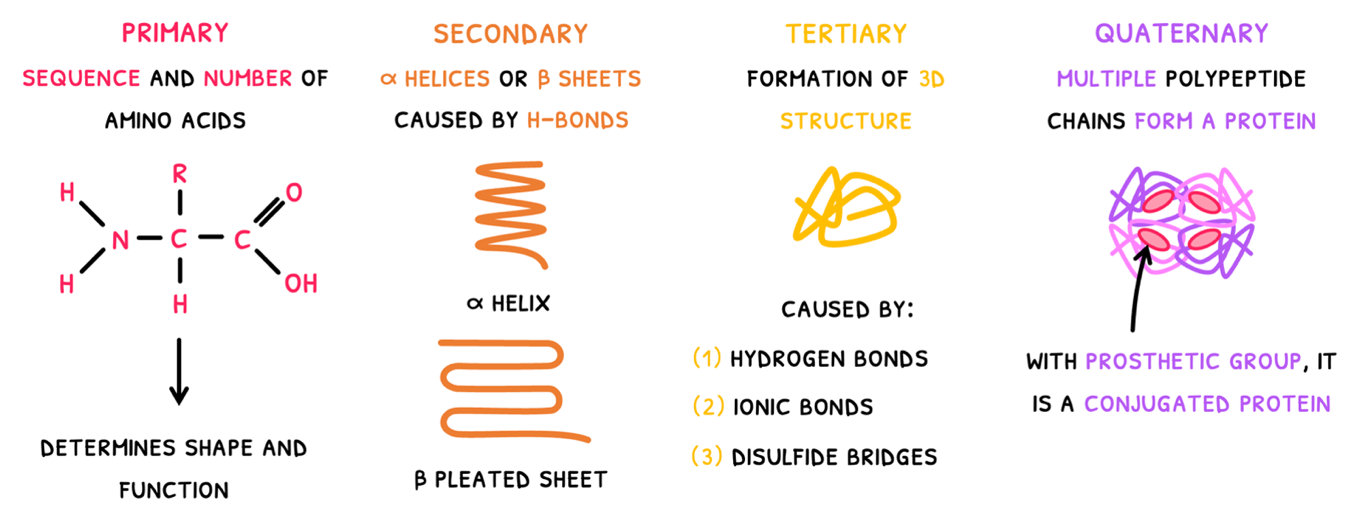
- Primary structure - this is simply the sequence and number of amino acids in the polypeptide chain. It ultimately determines the 3D shape and function of the protein. As a result, proteins have predictable and repeatable structures to attain a specific shape.
- Secondary structure - this is the first level of folding where carboxyl and amine groups of amino acids form hydrogen bonds forming one of two structures:
- The α helix - which is spiral in shape.
- The β pleated sheet - which is made of parallel straight chains.
Tertiary structure - this is the second level of folding, where the polypeptide forms a 3D structure. It results from a number of bonds:
- Hydrogen bonds between H-NOF R-groups.
- Ionic bonds between charged R-groups. Amine and carboxyl groups can also participate in this by binding or releasing H+ ions to become charged.
- Disulfide bridges between cysteines.
- Dipole-dipole forces between polar R-groups. In soluble proteins, these are found on the outside of the protein, whilst in integral proteins, they are found on either end of the protein.
- London dispersion forces between non-polar R-groups. Since these are hydrophobic, they are clustered in the core of soluble proteins, but take up the middle region of an integral protein.
The tertiary structure is the final structure of some proteins, but many have an additional level of structure.
Quaternary structure - this is the combination of two or more polypeptide chains to form a final protein. Common examples include:
- Insulin - composed of two polypeptide chains bound together as a globular protein.
- Collagen - composed of three polypeptide chains twisted together as a fibrous protein.
- Haemoglobin - composed of four polypeptide chains huddled together as a globular protein.
Insulin and collagen are non-conjugated proteins, but haemoglobin is an example of a conjugated protein. Conjugated proteins possess a non-protein group, called a prosthetic group. In hemoglobin, each polypeptide chains contains each a haem group with Iron to bind with oxygen.
