Structure of water
Water is the most essential molecule needed to sustain life on Earth. Also known as dihydrogen monoxide, it is composed of two hydrogen atoms covalently bonded to an oxygen atom. The sharing of electrons in these bonds is unequal, which means that water is a polar molecule!
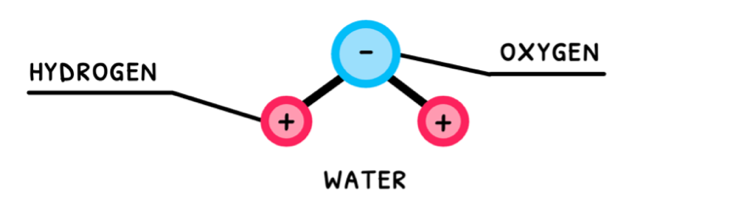
Polar molecules with hydrogen and one of nitrogen, oxygen, or fluorine exhibit hydrogen bonding. Water fits this description, and thus exhibits hydrogen bonding between its molecules. Water’s polarity and its hydrogen bonding are the reason it is so versatile and the primary molecule we search for when attempting to detect alien life.
Properties of water
For the IB, you are expected to understand that water has four main properties: cohesion, adhesion, thermal properties, and solvent properties. Let’s go through each of those in detail.
Cohesion is the ability of molecules within a substance to stick to one another due to intermolecular forces. In water, the molecules stay together via their hydrogen bonding, meaning they can form a continuous surface.
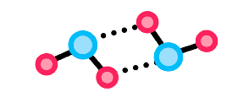
Examples of this are when cohesion forms one continuous water stream to travel in the xylem of plant stems or when it forms one continuous surface upon which insects can stand and walk.
Adhesion is the ability of molecules within a substance to stick to molecules of another substance due to intermolecular forces. Since water is polar and capable of hydrogen bonding, remember from Topic 2.1 that water will be able to form:
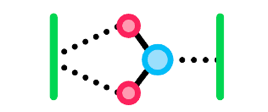
- Dipole-dipole forces with other charged, polar molecules, and hydrogen bonding with other H-NOF molecules. Molecules like this are considered hydrophilic since they would be attracted to water and able to bond with it.
- No strong intermolecular bonds with non-polar molecules, which would be considered hydrophobic since they would not be attracted to water and not bond with it.
An example of this is when adhesion sticks water to the polar cell walls of leaf cells to draw them out of xylem into the leaves.


